- Markovnikov's rule
-
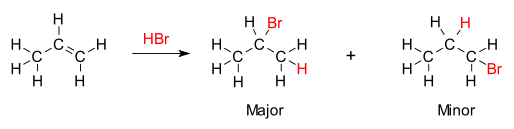 Markovnikov's rule is illustrated by the reaction of propene with hydrobromic acid
Markovnikov's rule is illustrated by the reaction of propene with hydrobromic acidIn organic chemistry, Markovnikov's rule or Markownikoff's rule is an observation based on Zaitsev's rule. It was formulated by the Russian chemist Vladimir Vasilevich Markovnikov in 1870.[1][2]
The rule states that with the addition of a protic acid HX to an alkene, the acid hydrogen (H) becomes attached to the carbon with fewer alkyl substituents, and the halide (X) group becomes attached to the carbon with more alkyl substituents. [3] [4]
The same is true when an alkene reacts with water in an addition reaction to form alcohol. The hydroxyl group (OH) bonds to the carbon that has the greater number of carbon-carbon bonds, while the hydrogen bonds to the carbon on the other end of the double bond, that has more carbon-hydrogen bonds.
The chemical basis for Markovnikov's Rule is the formation of the most stable carbocation during the addition process. The addition of the hydrogen (in the form of a proton) to one carbon atom in the alkene creates a positive charge on the other carbon, forming a carbocation intermediate. The more substituted the carbocation (the more bonds it has to carbon or to electron-donating substituents) the more stable it is, due to induction and hyperconjugation. The major product of the addition reaction will be the one formed from the more stable intermediate. Therefore, the major product of the addition of HX (where X is some atom more electronegative than H) to an alkene has the hydrogen atom in the less substituted position and X in the more substituted position. However, the other less substituted, less stable carbocation will still be formed to some degree, and will proceed to form the minor product with the opposite attachment of X.
The rule may be summarized as "the rich get richer (Jumi Shin)": a carbon rich in substituents will gain more substituents and the carbon with more hydrogens attached will get the hydrogen in many organic addition reactions.
Anti-Markovnikov rule
Mechanisms which avoid the carbocation intermediate may react through other mechanisms that are regioselective, against what Markovnikov's rule predicts, such as free radical addition. Such reactions are said to be anti-Markovnikov, since the halogen adds to the less substituted carbon, exactly the opposite of Markovnikov reaction. Again, like the positive charge, the radical is most stable when in the more substituted position
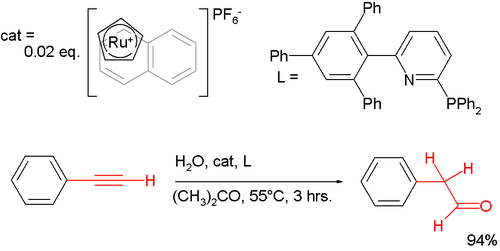
Anti-Markovnikov behaviour extends to other chemical reactions than just additions to alkenes. One anti-Markovnikov manifestation is observed in hydration of phenylacetylene that, gold-catalyzed, gives regular acetophenone but with a special ruthenium catalyst[5] the other regioisomer 2-phenylacetaldehyde:[6]
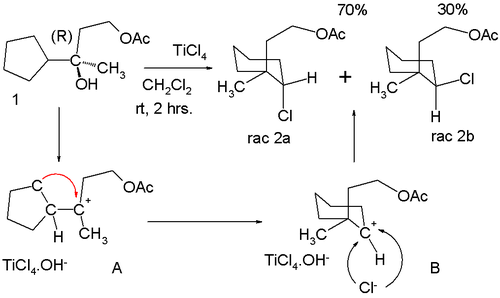
Anti-Markovnikov behaviour can also manifest itself in certain rearrangement reactions. In a titanium(IV) chloride catalyzed formal nucleophilic substitution at enantiopure 1 in the scheme below, two racemic products are formed 2a and 2b:[7]
This product distribution can be rationalized by assuming that loss of the hydroxy group in 1 gives the tertiary carbocation A which rearranges to the seemingly less attractive secondary carbocation B. Chlorine can approach this center from two faces leading to the observed mixture of isomers.
Another famous example of anti-Markovnikov addition is hydroboration.
External links
Khan academy lecture http://www.khanacademy.org/video/markovnikov-s-rule-and-carbocations
References
- ^ W. Markownikoff (1870). "Ueber die Abhängigkeit der verschiedenen Vertretbarkeit des Radicalwasserstoffs in den isomeren Buttersäuren". Annalen der Pharmacie 153 (1): 228–259. doi:10.1002/jlac.18701530204.
- ^ Was Markovnikov’s Rule an Inspired Guess? Peter Hughes 1152 Journal of Chemical Education • Vol. 83 No. 8 August 2006
- ^ Additions to Alkenes: Regiochemistry
- ^ Organic Chemistry, 6th Edition, by John McMurry. Section 6.9, page 187
- ^ cat;-0;'9-'09-alyst system based on in-situ reaction of ruthenocene with Cp and naphthalene ligands and a second bulky pyridine ligand
- ^ Highly Active in Situ Catalysts for Anti-Markovnikov Hydration of Terminal AlkynesAurélie Labonne, Thomas Kribber, and Lukas Hintermann Org. Lett.; 2006; 8(25) pp 5853 - 5856; (Letter) doi:10.1021/ol062455k
- ^ TiCl4 Induced Anti-Markovnikov Rearrangement Mugio Nishizawa, Yumiko Asai, and Hiroshi Imagawa Org. Lett.; 2006; 8(25) pp 5793 - 5796; (Letter) doi:10.1021/ol062337x.
Categories:- Physical organic chemistry
- Rules of thumb
Wikimedia Foundation. 2010.