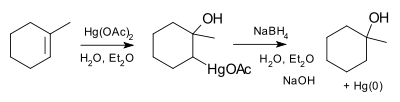
- Oxymercuration reaction
-
The oxymercuration reaction is an electrophilic addition organic reaction that transforms an alkene into a neutral alcohol. In oxymercuration, the alkene reacts with mercuric acetate (AcO-Hg-OAc) in aqueous solution to yield the addition of an acetoxymercuri (HgOAc) group and a hydroxy (OH) group across the double bond. Carbocations are not formed in this process and thus rearrangements are not observed. The reaction follows Markovnikov's rule (the hydroxy group will always be added to the more substituted carbon) and it is an anti addition (the two groups will be trans to each other).[2][3][4]
Oxymercuration followed by demercuration is called an oxymercuration-reduction reaction. This reaction, which is almost always done in practice instead of oxymercuration, is treated at the conclusion of the article.
Contents
Mechanism
Oxymercuration can be fully described in three steps(the whole process is sometimes called deoxymercuration), which is illustrated in stepwise fashion to the right. In the first step, the nucleophilic double bond attacks the mercury ion, ejecting an acetoxy group. The electron pair on the mercury ion in turn attacks a carbon on the double bond, forming a mercurinium ion in which the mercury atom bears a positive charge. The electrons in the highest occupied molecular orbital of the double bond are donated to mercury's empty dz2 orbital and the electrons in mercury's dxz orbital are donated in the lowest unoccupied molecular orbital of the double bond.
In the second step, the nucleophilic water molecule attacks the more substituted carbon, liberating the electrons participating in its bond with mercury. The electrons collapse to the mercury ion and neutralizes it. The oxygen in the water molecule now bears a positive charge.
In the third step, the negatively charged acetoxy ion that was expelled in the first step attacks a hydrogen of the water group, forming the waste product HOAc. The two electrons participating in the bond between oxygen and the attacked hydrogen collapse into the oxygen, neutralizing its charge and creating the final alcohol product.
Regioselectivity and stereochemistry
Oxymercuration is very regioselective and is a textbook Markovnikov reaction; ruling out extreme cases, the water nucleophile will always preferentially attack the more substituted carbon, depositing the resultant hydroxy group there. This phenomenon is explained by examining the three resonance structures of the mercurinium ion formed at the end of the step one.
By inspection of these structures, it is seen that the positive charge of the mercury atom will sometimes reside on the more substituted carbon (approximately 4% of the time). This forms a temporary tertiary carbocation, which is a very reactive electrophile. The nucleophile will attack the mercurinium ion at this time. Therefore, the nucleophile attacks the more substituted carbon because it retains more positive character than the lesser substituted carbon.
Stereochemically, oxymercuration is an anti addition. As illustrated by the second step, the nucleophile cannot attack the carbon from the same face as the mercury ion because of steric hindrance. There is simply insufficient room on that face of the molecule to accommodate both a mercury ion and the attacking nucleophile. Therefore, when free rotation is impossible, the hydroxy and acetoxymercuri groups will always be trans to each other.
Shown below is an example of regioselectivity and stereospecificity of the oxymercuration reaction with substituted cyclohexenes. A bulky group like t-butyl locks the ring in a chair conformation and prevents ring flips. With 4-t-butylcyclohexene, oxymercuration yields two products - where addition across the double bond is always anti - with slight preference towards acetoxymercury group trans to the t-butyl group, resulting in slightly more cis product. With 1-methyl-4-t-butylcyclohexene, oxymercuration yields only one product - still anti addition across the double bond - where water only attacks the more substituted carbon. The reason for anti addition across the double bond is to maximize orbital overlap of the lone pair of water and the empty orbital of the mercurinium ion on the opposite side of the acetoxymercury group. Regioselectivity is observed to favor water attacking the more substituted carbon, but water does not add syn across the double bond which implies that the transition state favors water attacking from the opposite side of the acetomercury group.[5]
Oxymercuration-reduction
In practice, the mercury adduct product created by the oxymercuration reaction is almost always treated with sodium borohydride (NaBH4) in aqueous base in a reaction called demercuration. In demercuration, the acetoxymercury group is replaced with a hydrogen in a stereochemically insensitive reaction known as reductive elimination. The combination of oxymercuration followed immediately by demercuration is called an oxymercuration-reduction reaction.[6]
 Mechanism for demercuration
Mechanism for demercurationTherefore, the oxymercuration-reduction reaction is the net addition of water across the double bond. Any stereochemistry set up by the oxymercuration step is scrambled by the demercuration step, so that the hydrogen and hydroxy group may be cis or trans from each other. Oxymercuration-reduction is a popular laboratory technique to achieve alkene hydration with Markovnikov selectivity while avoiding carbocation intermediates and thus the rearrangement which can lead to complex product mixtures.
Other applications
Oxymercuration is not limited to an alkene reacting with water. Using an alkyne instead of an alkene yields an enol, which tautomerizes into a ketone. Using an alcohol instead of water yields an ether. In both cases, Markovnikov's rule is observed.
Using a vinyl ether in the presence of an alcohol allows the transfer of the alkoxy group (RO-) from the alcohol to the ether. An allyl alcohol and a vinyl ether under the conditions of oxymercuration reaction can give R-CH=CH-CH2-O-CH=CH2, which is suitable for a Claisen Rearrangement.[7]
See also
- Hydroboration–oxidation reaction
- Alcohol
References
- ^ Organic Syntheses OS 6:766 Link
- ^ Loudon, Marc G. (2002). "Addition Reactions of Alkenes.". Organic Chemistry (Fourth Edition ed.). Oxford University Press. pp. 165–168.
- ^ McGraw-Hill Higher Education (2000). Oxymercuration-Demercuration of Alkenes. http://www.mhhe.com/physsci/chemistry/carey/student/olc/ch14oxymercurationdemercuration.html.
- ^ Schleifenbaum, Andreas (2001). "Oxymercuration.". Reaktionen, Reagenzien und Prinzipien. http://fachschaft.cup.uni-muenchen.de/~schleifi/reaktion/reaction/oxymercu.html.
- ^ Pasto, D. J.; Gontarz, J. A.. "Studies on the Mechanism of the Oxymercuration of Substituted Cyclohexenes.". Journal of the American Chemical Society (1971), 93, pg 6902-6908.
- ^ Bordwell, Frederick G.; Douglass, Miriam L. "Reduction of Alkylmercuric Hydroxides by Sodium Borohydride.". Journal of the American Chemical Society (1966), 88, pg 993-999.
- ^ McMurry, J. E.; Andrus A.; Ksander G. M.; Muesser, J. H.; Johnson, M. A.. "Stereospecific Total Synthesis of Aphidicolin.". Journal of the American Chemical Society (1979), 101, pg 1330-1332.
Categories:- Addition reactions
- Carbon-heteroatom bond forming reactions
Wikimedia Foundation. 2010.