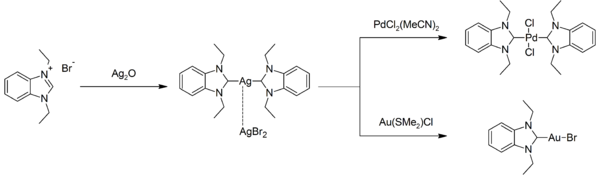- Organosilver chemistry
-
Organosilver chemistry in chemistry is the study of organometallic compounds containing a carbon to silver chemical bond and the study of silver as catalyst in organic reactions.[1][2][3] In the group 11 elements silver is the element below copper. The chemistries have much in common but organosilver catalysis is much less common (mostly academic study) than organocopper chemistry obviously due to the price of the metal but also due to poor thermal stability of the organocompounds. The oxidation state for silver in organosilver compounds in exclusively +1 with the notable exception of Ag(III) in the trifluoromethyl silver anion Ag(CF3)4- because of the electron-withdrawing effect of the trifluoromethyl groups. Poor thermal stability is reflected in decomposition temperatures of AgMe (-50°C) versus CuMe (-15°C) and PhAg (74°C) vs PhCu (100°C)
The first attempts in organosilver were recorded by Buckton in 1859[4] and by J. A. Wanklyn & L. Carius in 1861 [5]. The first synthesis of an organosilver compound (methyl silver) was described by Semerano and Riccoboni[6] in 1941.
Contents
Compounds
Phenylsilver can be obtained by reaction of silver nitrate with an trialkylphenyllead compound:
- AgNO3 + R3PhPb → PhAg
Alternatively, diphenylzinc may be used:[7]
- Ph2Zn + AgNO3 → PhAg + PhZnNO3
The silver mesitylene adduct is a tetramer. it can be formed from silver chloride and the Grignard reagent:[8]
- AgCl + MesMgBr → Ag4(Mes)4 + MgClBr
Silver forms stable complexes with ylides such as triphenylphosphonium methylide:
- AgCl + Ph3P=CH2 → AgCl(Ph3P=CH2)
The C-Ag bond is stabilized by perfluoroalkyl ligands:[9]
- AgF + CF2=CF(CF3) → AgCF(CF3)2
Alkenylsilver compounds are also more stable than their alkylsilver counterparts. Vinylsilver can be obtained by reaction of silver nitrate with tetravinyllead:[10]
- AgNO3 + (CH2=CH)4Pb → (CH2=CH)Ag + (CH2=CH)3PbNO3
Silver-NHC complexes are easily prepared, and are commonly used to prepare other NHC complexes by displacing labile ligands. For example, the reaction of the bis(NHC)silver(I) complex with bis(acetonitrile)palladium dichloride or chlorido(dimethyl sulfide)gold(I):[11]
Catalysis
In catalysis silver is active as silver oxide in the Wolff rearrangement. Silver nitrate is used to separate out alkenes as the η2-alkene complex. Silver is also present in other carbon-carbon bond skeletal rearrangements such as the quadricyclane to norbornadiene rearrangement, the cubane to cuneane rearrangement and the rearrangement of the cyclobutadiene dimer to cyclooctatetraene.
See also
- Chemical bonds of carbon with other elements in the periodic table:
CH He CLi CBe CB CC CN CO CF Ne CNa CMg CAl CSi CP CS CCl CAr CK CCa CSc CTi CV CCr CMn CFe CCo CNi CCu CZn CGa CGe CAs CSe CBr CKr CRb CSr CY CZr CNb CMo CTc CRu CRh CPd CAg CCd CIn CSn CSb CTe CI CXe CCs CBa CHf CTa CW CRe COs CIr CPt CAu CHg CTl CPb CBi CPo CAt Rn Fr Ra Rf Db Sg Bh Hs Mt Ds Rg Cn Uut Uuq Uup Uuh Uus Uuo ↓ CLa CCe CPr CNd CPm CSm CEu CGd CTb CDy CHo CEr CTm CYb CLu Ac Th Pa CU Np Pu Am Cm Bk Cf Es Fm Md No Lr Chemical bonds to carbon Core organic chemistry Many uses in chemistry Academic research, but no widespread use Bond unknown / not assessed References
- ^ W.A. Herrmann, ed (1999). Synthetic Methods of Organometallic and Inorganic Chemistry. 5, Copper, Silver, Gold, Zinc, Cadmium, and Mercury. Stuttgart: Thieme. ISBN 3-13-103061-5.
- ^ Christoph Elschenbroich (2006). Organometallics (3 ed.). Weinheim: Wiley-VCH. ISBN 3-527-29390-6.
- ^ The Chemistry of Organic Derivatives of Gold and Silver. Edited by Saul Patai and Zvi Rappoport Copyright 1999 John Wiley & Sons, Ltd. ISBN: 0-471-98164-8
- ^ Buckton, G. B. (1859). "Untersuchungen über organische Metallverbindungen". Annalen der Chemie und Pharmacie 109 (2): 218–227. doi:10.1002/jlac.18591090216.
- ^ Wanklyn, J. A.; Carius, L. (1861). "10. Ueber eine neue Wasserstoffverbindung des Eisens". Annalen der Chemie und Pharmacie 120 (1): 69. doi:10.1002/jlac.18611200107.
- ^ Semerano, G.; Riccoboni, L. (1941). "Beitrag zur Kenntnis der metallorganischen Verbindungen, I. Mitteil.: Silbermethyl, Silber-äthyl und Silber-n-propyl". Berichte der deutschen chemischen Gesellschaft (A and B Series) 74 (7): 1089. doi:10.1002/cber.19410740703.
- ^ Boersma, J; Des Tombe, F.J.A.; Weijers, F.; Van Der Kerk, G.J.M. (1977). "A new, easy synthesis of phenylsilver". J. Organomet. Chem. 124 (2): 229. doi:10.1016/S0022-328X(00)90970-7.
- ^ Meyer, Edouard Marc.; Gambarotta, Sandro.; Floriani, Carlo.; Chiesi-Villa, Angiola.; Guastini, Carlo. (1989). "Polynuclear aryl derivatives of Group 11 metals. Synthesis, solid state-solution structural relationship, and reactivity with phosphines". Organometallics 8 (4): 1067–1079. doi:10.1021/om00106a031.
- ^ Miller, W. T.; Burnard, R. J. (1968). "Perfluoroalkylsilver compounds". J. Am. Chem. Soc. 90 (26): 7367–7368. doi:10.1021/ja01028a047.
- ^ Holliday, A; Pendlebury, R.E. (1967). "Vinyllead compounds I. Cleavage of vinyl groups from tetravinyllead". J. Organomet. Chem. 7 (2): 281–284. doi:10.1016/S0022-328X(00)91078-7.
- ^ Wang, Harrison M. J.; Lin, Ivan J. B. (1998). "Facile Synthesis of Silver(I)−Carbene Complexes. Useful Carbene Transfer Agents". Organometallics 17 (5): 972. doi:10.1021/om9709704.
Categories:- Silver compounds
- Organometallic compounds
Wikimedia Foundation. 2010.