- Organotellurium chemistry
-
Organotellurium chemistry in chemistry describes the synthesis and properties of chemical compounds containing a carbon to tellurium chemical bond.[1] Organotellurium chemistry was developed in the wake of organoselenium chemistry and, sharing the same group in the periodic table, both chemistries have much in common. The field receives academic interest but organotellurium compounds rarely venture outside the laboratory. Dimethyl telluride is the only organotellurium compound that has been quantified in environmental samples.[2]
Diphenyl ditelluride is used as a TePh source in organic synthesis. Dimethyl telluride as a product of microbial metabolism first discovered in 1939. it is used in Metalorganic vapour phase epitaxy. Commonly used tellurium based reagents are hydrogen telluride, NaHTe, sodium telluride, PhTeH and PhTeNa.
Reactions with these reagents are:
- Organic reduction of aldehydes, alkenes, alkynes, nitro compounds, oxiranes to alkenes
- Debromination of vicinal dibromides with E2 elimination
- Organic oxidation of by compounds of the type (ArTeO)2O
- Tellurium tetrachloride reacts with alkenes and alkynes to the chloro tellurium trichloride addition product.
- Tellurium in vinylic tellurium trichlorides can be replaced by halides with a variety of reagents (iodine, NBS )
- Detellurative cross-coupling reaction: Compounds of the type Ar2TeCl2 engage in a coupling reaction to the corresponding biaryls with Raney nickel or palladium [3]
- Another type is this Stille reaction [4]:
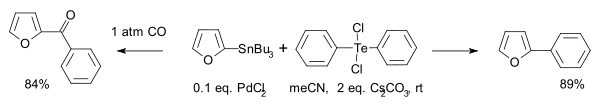
- Hydrotelluration: Compounds of the type RTeH react with alkynes R'CCH to R'HCCTeR with anti addition to a Z-alkene. In contrast hydrostannylation, hydrozirconation and hydroalumination in similar reactions react with syn addition.
- Te/Li exchange in transmetallation [5] is used in the synthesis of lithium reagents with demanding functional groups.
- Allylic oxidation: like the selenium counterpart selenoxide oxidation, allylic telluroxides undergo [2,3]-sigmatropic rearrangements forming allylic alcohols after hydrolysis.
- Olefin synthesis: Like the selenium counterpart selenoxide elimination, certain telluroxides (RTeOR) can form alkenes on heating.
See also
- The chemistry of carbon bonded to other elements in the periodic table:
CH He CLi CBe CB CC CN CO CF Ne CNa CMg CAl CSi CP CS CCl CAr CK CCa CSc CTi CV CCr CMn CFe CCo CNi CCu CZn CGa CGe CAs CSe CBr CKr CRb CSr CY CZr CNb CMo CTc CRu CRh CPd CAg CCd CIn CSn CSb CTe CI CXe CCs CBa CHf CTa CW CRe COs CIr CPt CAu CHg CTl CPb CBi CPo CAt Rn Fr Ra Rf Db Sg Bh Hs Mt Ds Rg Cn Uut Uuq Uup Uuh Uus Uuo ↓ CLa CCe CPr CNd CPm CSm CEu CGd CTb CDy CHo CEr CTm CYb CLu Ac Th Pa CU Np Pu Am Cm Bk Cf Es Fm Md No Lr Chemical bonds to carbon Core organic chemistry Many uses in chemistry Academic research, but no widespread use Bond unknown / not assessed References
- ^ Organometallic Reagents for Synthetic Purposes: Tellurium Nicola Petragnani, and Wai-Ling Lo J. Braz. Chem. Soc., Vol. 9, No. 5, 415-425, 1998 http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0103-50531998000500002.
- ^ Wallschläger, D.; Feldmann, F. (2010). Formation, Occurrence, Significance, and Analysis of Organoselenium and Organotellurium Compounds in the Environment. Metal Ions in Life Sciences. 7, Organometallics in Environment and Toxicology. RSC Publishing. pp. 319–364. ISBN 978-1-84755-177-1.
- ^ For an example see: Organic Syntheses, Coll. Vol. 6, p.468 (1988); Vol. 57, p.18 (1977). http://orgsynth.org/orgsyn/pdfs/CV6P0468.pdf
- ^ Palladium- and copper-catalyzed cross-coupling and carbonylative cross-coupling of organotellurium compounds with organostannanes (Chem. Commun. 1999, 2117) - Royal Society of Chemistry Suk-Ku Kang, Sang-Woo Lee and Hyung-Chul Ryu Link
- ^ For an example see: Organic Syntheses, Coll. Vol. 9, p.234 (1998); Vol. 72, p.154 (1995). http://orgsynth.org/orgsyn/pdfs/CV9P0234.pdf
Categories:- Organotellurium compounds
Wikimedia Foundation. 2010.
