- Dimethyl telluride
-
Dimethyl telluride 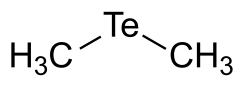
 Systematic nameOther namesDimethyltellane[1]
Systematic nameOther namesDimethyltellane[1]Identifiers CAS number 593-80-6 
PubChem 68977 ChemSpider 62199 
EC number 209-809-5 KEGG C02677 
MeSH dimethyltelluride ChEBI CHEBI:4613 
Beilstein Reference 1696849 Gmelin Reference 1480 Jmol-3D images Image 1 - C[Te]C
Properties Molecular formula C2H6Te Molar mass 157.67 g mol−1 Exact mass 159.953172945 g mol-1 Appearance Pale yellow, translucent liquid Odor Garlic Melting point -10 °C, 263 K, 14 °F
Boiling point 82 °C, 355 K, 180 °F
Related compounds Related compounds Dimethylmercury
 telluride (verify) (what is:
telluride (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Dimethyl telluride is an organotelluride compound, formula (CH3)2Te, also known by the abbreviation DMTe.
This was the first material used to grow epitaxial cadmium telluride and mercury cadmium telluride using metalorganic vapour phase epitaxy.[2][3]
Dimethyl telluride as a product of microbial metabolism was first discovered in 1939.[4] Dimethyl telluride is produced by some fungi and bacteria (Penicillium brevicaule, P. chrysogenum, and P. notatum and the bacterium Pseudomonas fluorescens).[5]
The toxicity of DMTe is unclear. It is produced by the body when tellurium or one of its compounds are ingested. It is noticeable by its garlic-like smell (resembles rotting garlic in the absence of air). Tellurium is known to be toxic.[6]
References
- ^ a b "dimethyl telluride (CHEBI:4613)". Chemical Entities of Biological Interest (ChEBI). UK: European Bioinformatics Institute. 25 September 2006. IUPAC Names. https://www.ebi.ac.uk/chebi/searchId.do?chebiId=4613. Retrieved 19 September 2011.
- ^ J. Tunnicliffe, S. J. C. Irvine, O. D. Dosser, J. B. Mullin (1984). "A new MOVPE technique for the growth of highly uniform CMT". J. Cryst. Growth. 68 (1): 245–253. doi:10.1016/0277-5387(95)00249-X.
- ^ H. B. Singh, N. Sudha (1996). "Organotellurium precursors for metal organic chemical vapour deposition (MOCVD) of mercury cadmium telluride (MCT)". Polyhedron 15 (5–6): 745–763. doi:10.1016/0277-5387(95)00249-X.
- ^ M. L. Bird, F. Challenger (1939). "Formation of organometalloidal and similar compounds by microorganisms. VII. Dimethyl telluride". Journal of the Chemical Society 163-168: 299–305. doi:10.1039/JR9390000163.
- ^ R. S. T. Basnayake, J. H. Bius, O. M. Akpolat, T. G. Chasteen (2001). "Production of dimethyl telluride and elemental tellurium by bacteria amended with tellurite or tellurate". Applied Organometallic Chemistry 15 (6): 499–510. doi:10.1002/aoc.186.
- ^ T. G. Chasteen, R. Bentley (2003). "Biomethylation of Selenium and Tellurium: Microorganisms and Plants". Chemical Reviews 103 (1): 1–26. doi:10.1021/cr010210. PMID 12517179.
- Escherichia coli TehB Requires S-Adenosylmethionine as a Cofactor To Mediate Tellurite Resistance, Mingfu Liu, R. J. Turner, T. L. Winstone, A. Saetre, M. Dyllick-Brenzinger, G. Jickling, L. W. Tari, J. H. Weiner, and D. E. Taylor, Journal of Bacteriology, Vol. 182, No. 22 pp. 6509-6513 (2000)
- Vacuum ultraviolet absorption spectra of dimethyl sulfide, dimethyl selenide, and dimethyl telluride, J. D. Scott, G. C. Causley, and B. R. Russell, The Journal of Chemical Physics, Vol. 59, Iss. 12, pp. 6577-6586 (1973) doi:10.1063/1.1680037
- M. M. Gharieb, M. Kierans, G. M. Gadd (1999). "Transformation and tolerance of tellurite by filamentous fungi: accumulation, reduction, and volatilization". Mycological Research 103 (3): 299–305. doi:10.1017/S0953756298007102.
External links
- Epichem (Commercial supplier datasheet)
Categories:- Tellurides
- Organotellurium compounds
Wikimedia Foundation. 2010.
