- Dimethylmercury
-
Dimethylmercury 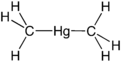
 DimethylmercurySystematic nameDimethylmercuryOther namesDimethyl mercury
DimethylmercurySystematic nameDimethylmercuryOther namesDimethyl mercuryIdentifiers CAS number 593-74-8 
PubChem 11645 ChemSpider 11155 
UNII C60TQU15XY 
EC number 209-805-3 UN number 3383 MeSH Dimethyl+mercury ChEBI CHEBI:30786 
RTECS number OW3010000 Beilstein Reference 3600205 Gmelin Reference 25889 Jmol-3D images Image 1
Image 2- C[Hg]C
[Hg](C)C
Properties Molecular formula C2H6Hg Molar mass 230.66 g mol−1 Exact mass 232.017575796 g mol-1 Appearance Colorless liquid Density 2.961 g cm-3 Melting point -43 °C, 230 K, -45 °F
Boiling point 93-94 °C, 366-367 K, 199-201 °F
Hazards EU Index 080-007-00-3 EU classification  T+
T+ N
NR-phrases R26/27/28, R33, R50/53 S-phrases (S1/2), S13, S28, S36, S45, S60, S61 NFPA 704 Flash point 5 °C  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Dimethylmercury ((CH3)2Hg) is an organomercury compound. This colorless liquid is one of the strongest known neurotoxins. It is described as having a slightly sweet smell, although inhaling enough vapor to detect its odor would be hazardous.
Contents
Synthesis, structure, reactions
The compound was one of the earliest organometallic complexes reported, reflecting its considerable stability. It is formed by treating sodium amalgam with methyl halides:
It can also be obtained by alkylation of mercuric chloride with methyllithium. The molecule adopts a linear structure with Hg-C bond lengths of 2.083 Å.[1]
Reactions
The most striking feature of the compound is its nonreactivity toward water. The corresponding organocadmium and organozinc compounds hydrolyze rapidly. The difference reflects the low affinity of Hg(II) for oxygen ligands. The compound reacts with mercuric chloride to give the mixed chloro-methyl compound:
- (CH3)2Hg + HgCl2 → 2 CH3HgCl
Whereas dimethylmercury is a volatile liquid, CH3HgCl is a crystalline solid.
Use
Dimethylmercury has almost no applications because of the risks involved. In toxicology, it is used as a reference toxin. It has also been used to calibrate NMR instruments for detection of mercury, although less toxic mercury salts are preferred.[2][3]
Safety
Dimethylmercury is extremely dangerous. Absorption of doses as low as 0.1 mL has proven fatal.[4] The risks are enhanced because of the high vapor pressure of the liquid.
Dimethylmercury passes through latex, PVC, butyl, and neoprene rapidly (within seconds) and is absorbed through the skin. Therefore, most laboratory gloves do not provide adequate protection from it, and the only safe precaution is to handle dimethylmercury while wearing highly resistant laminated gloves underneath long-cuffed neoprene or other heavy-duty gloves. A long face shield and work under a fume hood are also indicated.[4][5]
Dimethylmercury crosses the blood–brain barrier easily, probably owing to formation of a complex with cysteine. It is eliminated from the organism slowly, and therefore has a tendency to bioaccumulate. The symptoms of poisoning may be delayed by months, possibly too late for effective treatment.
The toxicity of dimethylmercury was highlighted with the death of the inorganic chemist Karen Wetterhahn of Dartmouth College in 1997, months after spilling no more than a few drops of this compound on her latex-gloved hand.[4]
See also
References
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ^ Chris Singer (1998-03-10). "199Hg Standards". http://www.chem.northwestern.edu/~ohallo/HgNMRStandards/. Retrieved 2009-05-03.
- ^ Roy Hoffman (2007-02-21). "Mercury NMR". http://chem.ch.huji.ac.il/nmr/techniques/1d/row6/hg.html. Retrieved 2009-05-03.
- ^ a b c Hazard Information Bulletin - Dimethylmercury. OSHA Safety and Health Information Bulletins (SHIBs), 1997-1998
- ^ Simon Cotton, Dimethylmercury and mercury poisoning. The Karen Wetterhahn story. Molecule of the Month.
External links
Categories:- Neurotoxins
- Organomercury compounds
- C[Hg]C
Wikimedia Foundation. 2010.

