- Organogermanium compound
-
Organogermanium compounds are organometallic compounds containing a carbon to germanium or hydrogen to germanium chemical bond. Organogermanium chemistry is the corresponding chemical science.[1] Germanium shares group 14 in the periodic table with silicon, tin and lead and not surprisingly the chemistry of organogermanium is in between that of organosilicon compounds and organotin compounds.
The main reason why the organogermanium is of limited synthetic value are the costs of germanium compounds. On the other hand germanium is advocated as a non-toxic alternative to many toxic organotin reagents and compounds like tetramethylgermanium and tetraethylgermanium are used in the microelectronics industry as precursors for germanium dioxide chemical vapor deposition.
The first organogermanium compound, tetraethylgermane, was synthesised by Winkler in 1887, by the reaction of germanium tetrachloride with diethylzinc.[2] The organogermanium compound bis (2-Carboxyethylgermanium)sesquioxide was first reported in 1966.[3]
Contents
Organogermanes
Organogermanes of the type R4Ge with alkyl (R) groups are accessed through the cheapest available germanium precursor germanium tetrachloride and alkyl nucleophiles. The following trends are observed going down the carbon group: The nucleophilicity increases Si<Ge<Sn as well as the hyperconjugation effect known as the beta-silicon effect Si<Ge<<Sn. Where the Si-C bond is mainly ionic and the Sn-C bond is mainly radical, bonds with germanium are in between.
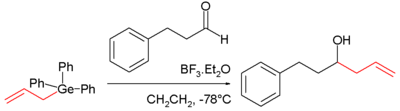
Just as with silicon the nucleophilicity of allyl germanes is high due to the intrinsic polarization of the bond (difference in electronegativity 2.55 − 2.01 = 0.54) and the combined stabilizing effect on the α-carbonion by the allyl group and the germanium atom. The germanium pendant of the Sakurai reaction was discovered in 1986:
The carbonyl group in this reaction is activated with boron trifluoride.
Germanium hydrides
Isobutylgermane (IBGe) (Me2CHCH2)GeH3 is the organogermanium hydride that is a high vapor pressure liquid germanium source for MOVPE. Isobutylgermane is currently investigated as safer and less hazardous alternative to toxic germane gas in microelectonic applications.
Tris(trimethylsilyl)germanium hydride (Me3Si)3GeH has been investigated as a non-toxic alternative to many tin hydrides such as tributyltinhydride.
Other germanium compounds
Many germanium reactive intermediates are known: germylenes (carbene pendants), germyl free radicals, germynes (carbyne pendants).[4]
As with silicon and contrasting with carbon, compounds containing Ge-C (germenes) and Ge-Ge (digermylenes) double bonds are unstable but known for instance the benzene pendant germanabenzene.
External links
- Tetramethylgermanium Datasheet commercial supplier
- Tetraethylgermanium Datasheet commercial supplier
- Tris(trimethylsilyl)germanium hydride Datasheet commercial supplier
See also
- Compounds of carbon with other elements in the periodic table:
CH He CLi CBe CB CC CN CO CF Ne CNa CMg CAl CSi CP CS CCl CAr CK CCa CSc CTi CV CCr CMn CFe CCo CNi CCu CZn CGa CGe CAs CSe CBr CKr CRb CSr CY CZr CNb CMo CTc CRu CRh CPd CAg CCd CIn CSn CSb CTe CI CXe CCs CBa CHf CTa CW CRe COs CIr CPt CAu CHg CTl CPb CBi CPo CAt Rn Fr Ra Rf Db Sg Bh Hs Mt Ds Rg Cn Uut Uuq Uup Uuh Uus Uuo ↓ CLa CCe CPr CNd CPm CSm CEu CGd CTb CDy CHo CEr CTm CYb CLu Ac Th Pa CU Np Pu Am Cm Bk Cf Es Fm Md No Lr Chemical bonds to carbon Core organic chemistry Many uses in chemistry Academic research, but no widespread use Bond unknown / not assessed References
- ^ Main Group Metals in Organic Synthesis, Hisashi Yamamoto (Editor), Koichiro Oshima (Editor) ISBN 3-527-30508-4 2004
- ^ Winkler, Clemens (1887). "Mittheilungen über des Germanium. Zweite Abhandlung". J. Prak. Chemie 36: 177–209. doi:10.1002/prac.18870360119. http://gallica.bnf.fr/ark:/12148/bpt6k90799n/f183.table. Retrieved 2008-08-20.
- ^ "Bis(2-carboxyethylgermanium(IV) sesquioxide". http://www.germaniumsesquioxide.com/Pages/History%20Germanium%20Sesquioxide.html.
- ^ Reactive intermediates in organogermanium chemistry Jacques Satge Pure & Appl. Chem., Vol.56, No.1, pp.137—150, 1984.
Categories:- Organogermanium compounds
Wikimedia Foundation. 2010.