- Organoiron chemistry
-
Organoiron chemistry is the chemistry of organometallic compounds containing a carbon-to-iron chemical bond.[1][2] Organoiron compounds are relevant in organic synthesis as reagents such as iron pentacarbonyl, diiron nonacarbonyl and disodium tetracarbonylferrate. Iron adopts oxidation states from Fe(-II) through to Fe(IV). Although iron is generally less active in many catalytic applications, it is less expensive and "greener" than other metals.[3] Organoiron compounds feature a wide range of ligands that support the Fe-C bond; as with other organometals, these supporting ligands prominently include phosphines, carbon monoxide, and cyclopentadienyl, but hard ligands such as amines are employed as well.
Contents
Iron carbonyls
The binary carbonyls and their anions
Important iron carbonyls are the three neutral binary carbonyls, iron pentacarbonyl, diiron nonacarbonyl, and triiron dodecacarbonyl. One or more carbonyl ligands in these compounds can be replaced by a variety of other ligands (dienes, phosphines).
Iron carbonyls have been used in stoichiometric carbonylation reactions, e.g. for the conversion of alkyl bromides to aldehydes. Disodium tetracarbonylferrate, "Collman's Reagent," can be alkylated followed by carbonylation to give the acyl derivatives that undergo protonolysis to afford aldehydes:
- LiFe(CO)4(C(O)R) + H+ → RCHO (+ iron containing products)
Similar iron acyls can be accessed by treating iron pentacarbonyl with organolithium compounds:
- ArLi + Fe(CO)5 → LiFe(CO)4C(O)R
In this case, the carbanion attacks a CO ligand. In a complementary reaction, Collman's reagent can be used to convert acyl chlorides to aldehydes. Similar reactions can be achieved with [HFe(CO)4]- salts.[4]
(Diene)Fe(CO)3 derivatives
Iron diene complexes are usually prepared from Fe(CO)5 or Fe2(CO)9. Derivatives are known for common dienes are cyclohexadiene, norbornadiene and cyclooctadiene, but even cyclobutadiene can be stabilized. In the complex with butadiene, the diene adopts a cis-conformation. Iron carbonyls are used as a protective group for dienes in hydrogenations and Diels-Alder reactions. Cyclobutadieneiron tricarbonyl is prepared from 3,4-dichlorocyclobutene and Fe2(CO)9.
Cyclohexadienes, many derived from Birch reduction of aromatic compounds, form derivatives (diene)Fe(CO)3. The affinity of the Fe(CO)3 unit for conjugated dienes is manifested in the ability of iron carbonyls catalyse the isomerisations of 1,5-cyclooctadiene to 1,3-cyclooctadiene. Cyclohexadiene complexes undergo hydride abstraction to give cyclohexadienyl cations, which add nucleophiles.[5]
The enone complex (benzylideneacetone)iron tricarbonyl serves as a source of the Fe(CO)3 subunit and is employed to prepare other derivatives. It is used complementarily to Fe2</sub(CO)9.
Alkynes form many compounds with upon reaction with iron carbonyls. These include cyclobutadiene derivatives, ferroles" of the formula Fe2(C4R4)(CO)6, as well as cyclopentadienone and cyclobutadiene derivatives.
Sulfur and phosphorus derivatives
Complexes of the type Fe2(SR)2(CO)6 and Fe2(PR2)2(CO)6 form, usually by the reaction of thiols and secondary phosphines with iron carbonyls.[6] The thiolates can also be obtained from the tetrahedrane Fe2S2(CO)6.
Cyclopentadienyl derivatives, including ferrocenes
Ferrocene and its derivatives
The rapid growth of organometallic chemistry in the 20th Century can be traced to the discovery of ferrocene, a very stable that foreshadowed the synthesis of many related sandwich compounds. Ferrocene is formed by reaction of sodium cyclopentadienyl with iron chloride:
- 2 NaC5H5 + FeCl2 → Fe(C5H5)2 + 2 NaCl
Ferrocene displays diverse reactivity localized on the cyclopentadienyl ligands, including Friedel-Crafts reactions and lithation. Ferrocene is also an structurally unusual scaffold as illustrated by the popularity of ligands such as 1,1'-bis(diphenylphosphino)ferrocene, which are useful in catalysis.[7] Treatment of ferrocene with aluminium trichloride and benzene gives the cation [CpFe(C6H6)]+. Oxidation of ferrocene gives the blue 17e species ferrocenium.
Fp2 and its derivatives
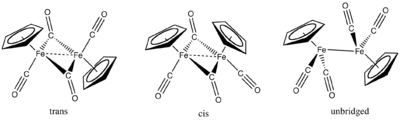
Fe(CO)5 reacts with dicyclopentadiene to give the cyclopentadienyl complex cyclopentadienyliron dicarbonyl dimer ([FeCp(CO)2]2). Reduction of this species with sodium gives "NaFp" (Fp = [FeCp(CO)2]-), a potent nucleophile and precursor to many derivatives of the type CpFe(CO)2R.[8] The derivative [FpCH2S(CH3)2]+ has been used in cyclopropanations.[9] FpAcyl compounds are prochiral, and studies have exploited the chiral derivatives CpFe(PPh3)(CO)acyl.[10] Pyrolysis of Fp2 gives the cuboidal cluster [FeCp(CO)]4.
Polyhapto organic ligands
Stable complexes with iron with and without CO ligands are known for a wide variety of polyunsaturated hydrocarbons, e.g. cycloheptatriene, azulene, cyclooctatetraene (COT), and bullvalene. The compound Fe(COT)2 is well known [11], Fe3(COT)3 was described in 2009 as the reaction product of Fe(COT2 with a catalytic amount of an persistent carbene. It can be regarded as an organic version of triiron dodecacarbonyl [12]
Phosphine- and amine-Fe(II) complexes
As for other organometallic compounds, organoiron(II) complexes in the absence of Cp ligands are commonly complemented by tertiary diphosphines and to a lesser extent amine/imine ligands. Complexes of the type FeX2(diphosphine)2 figure prominently in this area, provided early examples of C-H bond activation, dihydrogen complexes, and dinitrogen complexes. Complexes derived from Schiff bases are active catalysts for olefin polymerization.[13]
Organoiron compounds in organic synthesis and homogeneous catalysis
Because of its low cost and low toxicity of its salts, iron is often employed as a stoichiometric reagent. Iron role as a catalyst in organic reactions is overshadowed by the related chemistries of cobalt and nickel. Some main categories are:
- Addition reactions for example the Aldol reaction and Michael reaction[14]
- Substitution notably coupling reactions. Ferric chloride is a well known catalyst in the Friedel–Crafts reaction. Compounds of the type [(η3-allyl)Fe(CO)4+X- are allyl cation synthons in allylic substitution [15][16][17]. Likewise the compound Ph(CO2)Fe+(η2-vinyl(OEt))BF4- is a masked vinyl cation[18]. Disodium tetracarbonylferrate can be regarded a CO dianion synthon.[19]
- Cycloadditions, for example cyclopropanation using CpFe(CO)2CH2S+(CH3)2BF4- [20]. Also Ene reaction [21]
- Hydrogenation and reduction
- isomerization reactions and rearrangement reactions
- Cross-coupling reactions. Iron compounds such as Fe(acac)3 catalyze a wide range of cross-coupling reactions with one substrate an aryl or alkyl Grignard and the other substrate an aryl, alkenyl (vinyl), or acyl organohalide. In the related Kumada coupling the catalysts are based on palladium and nickel.
Biochemistry
In the area of bioorganometallic chemistry, organoiron species are found at the active sites of the three hydrogenase enzymes as well as carbon monoxide dehydrogenase.
See also
- Chemical bonds of carbon with other elements in the periodic table:
CH He CLi CBe CB CC CN CO CF Ne CNa CMg CAl CSi CP CS CCl CAr CK CCa CSc CTi CV CCr CMn CFe CCo CNi CCu CZn CGa CGe CAs CSe CBr CKr CRb CSr CY CZr CNb CMo CTc CRu CRh CPd CAg CCd CIn CSn CSb CTe CI CXe CCs CBa CHf CTa CW CRe COs CIr CPt CAu CHg CTl CPb CBi CPo CAt Rn Fr Ra Rf Db Sg Bh Hs Mt Ds Rg Cn Uut Uuq Uup Uuh Uus Uuo ↓ CLa CCe CPr CNd CPm CSm CEu CGd CTb CDy CHo CEr CTm CYb CLu Ac Th Pa CU Np Pu Am Cm Bk Cf Es Fm Md No Lr Chemical bonds to carbon Core organic chemistry Many uses in chemistry Academic research, but no widespread use Bond unknown / not assessed References
- ^ Synthesis of Organometallic Compounds: A Practical Guide Sanshiro Komiya Ed. S. Komiya, M. Hurano 1997
- ^ Iron-Catalyzed Reactions in Organic SynthesisCarsten Bolm, Julien Legros, Jacques Le Paih, and Lorenzo Zani Chem. Rev. 2004, 104, 6217-6254 {{DOI:10.1021/cr040664h}}
- ^ Enthaler, S.; Junge, K. and Beller, M., "Sustainable Metal Catalysis with Iron: From Rust to a Rising Star?" Angew. Chem. Int. Ed., 2008, 47, 3317-3321.
- ^ J.J. Brunet “Tetracarbonylhydridoferrates, MHFe(CO)4: Versatile Tools in Organic Synthesis and Catalysis” Chem. Rev. 1990, volume 90, 1041-1059 1041. doi:10.1021/cr00104a006
- ^ A. J. Birch and K. B. Chamberlain (1973), "Tricarbonyl[(2,3,4,5-É≈)-2,4-cyclohexadien-1-oneiron and Tricarbonyl[(1,2,3,4,5-É≈)-2-methox-2,4-cyclohexadien-1-yl]iron(1+) Hexafluorophosphate(1-) from Anisole"], Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV6P0996; Coll. Vol. 6: 996
- ^ King, R. B., "Organosulfur Derivatives of Metal Carbonyls. I. The Isolation of Two Isomeric Products in the Reaction of Triiron Dodecacarbonyl with Dimethyl Disulfide", J. Am. Chem. Soc., 1962, 84, 2460.
- ^ Petr Stepnicka "Ferrocenes: Ligands, Materials and Biomolecules" J. Wiley, Hoboken, 2008. ISBN 0470035854
- ^ Keith H. Pannell and Hemant K. Sharma "(Cyclopentadienyl)dicarbonylmethyliron ((η5-C5H5)Fe(CO)2CH3, FpMe), a Seminal Transition-Metal Alkyl Complex: Mobility of the Methyl Group" Organometallics 2010 doi: 10.1021/om1004594
- ^ Organic Syntheses, Coll. Vol. 9, p.372 (1998); Vol. 70, p.177 (1992). Link
- ^ Karola Ruck-Braun “Iron Acyl Complexes” in Transition Metals for Organic Synthesis. Vol. 1. 2nd Ed., M. Beller, C. Bohn, Eds. Wiley-VCH, 2004, Weinheim. ISBN 3-527-30613-7.
- ^ D. H. Gerlach, R. A. Schunn, Inorg. Syn. volume 15, 2 (1974) doi:10.1002/9780470132463.ch1.
- ^ Carbenes As Catalysts for Transformations of Organometallic Iron Complexes Vincent Lavallo and Robert H. Grubbs Science 2009: Vol. 326. no. 5952, pp. 559 - 562 {{DOI:10.1126/science.1178919}}
- ^ Allan, L. E. N.; Shaver, M. P.; White, A. J. P. and Gibson, V. C., "Correlation of Metal Spin-State in alpha-Diimine Iron Catalysts with Polymerization Mechanism", Inorg. Chem., 2007, 46, 8963-8970.
- ^ Example: Organic Syntheses, Coll. Vol. 10, p.588 (2004); Vol. 78, p.249 (2002). Link
- ^ Example: Organic Syntheses, Coll. Vol. 10, p.672 (2004); Vol. 78, p.189 (2002). Link
- ^ See also Organic Syntheses, Coll. Vol. 6, p.1001 (1988); Vol. 57, p.16 (1977). Link
- ^ See also Organic Syntheses, Coll. Vol. 6, p.996 (1988); Vol. 57, p.107 (1977). Link
- ^ Organic Syntheses, Coll. Vol. 8, p.479 (1993); Vol. 66, p.95 (1988). Link
- ^ Organic Syntheses, Coll. Vol. 6, p.807 (1988); Vol. 59, p.102 (1979). Link
- ^ Organic Syntheses, Coll. Vol. 9, p.372 (1998); Vol. 70, p.177 (1992). Link
- ^ Organic Syntheses, Coll. Vol. 9, p.310 (1998); Vol. 71, p.167 (1993). Link
Categories:- Organoiron compounds
Wikimedia Foundation. 2010.