- Organorhodium chemistry
-
Organorhodium chemistry is the chemistry of organometallic compounds containing a carbon to rhodium chemical bond, and the study of rhodium and rhodium compounds as catalysts in organic reactions.[1]
Organometallic rhodium compounds share many characteristics with those of cobalt (see organocobalt compounds) in the same group 9. Rhodium can exist in oxidation states +IV to -III but +I and +III are the most common. Rhodium(I) compounds (d8 configuration) can occur with square planar geometries and with trigonal bipyramidal geometries. Important homoleptic rhodium compounds are tetrarhodium dodecacarbonyl Rh4(CO)10 and hexadecacarbonylhexarhodium Rh6(CO)16. The hexarhodium compound is less preferred due to poor solubility. Both are important catalysts in hydroformylation of alkenes often accompanied by a phosphine ligand:
Nitrobenzene reduction is another reaction catalysed by this compound type:
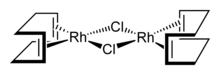
Cyclooctadiene rhodium chloride dimer [RhCl(COD)]2 is investigated for its use in C-H bond activation. Sandwich compounds of rhodium, such as rhodocene, and half-sandwich compounds like [(η5-Cp)Rh(CO)2] are well known.
Other relevant organorhodium compounds used as catalysts Name Molecular formula CAS (Acetylacetonato)dicarbonylrhodium(I) Rh(CO)2(C5H7O2) 14874-82-9 Bicyclo[2.2.1]hepta-2,5-diene-rhodium(I) chloride dimer C14H16Cl2Rh2 12257-42-0 Chloro(1,5-cyclooctadiene)rhodium(I) dimer C16H24Cl2Rh2 12092-47-6 (Acetylacetonato)(norbornadiene)rhodium(I) C12H15O22Rh 32354-50-0 Chloro(1,5-hexadiene)rhodium(I),dimer C12H24Rh2Cl2 32965-49-4 Acetylacetonatobis(ethylene)rhodium(I) C9H15O2Rh 12082-47-2 [1,4-Bis(diphenylphosphino)butane](1,5-cyclooctadiene)rhodium(I) tetrafluoroborate C36H40BF4P2Rh 79255-71-3 Cyclometallation
Cyclometalated rhodium compounds constitute an important class of organometallic chemistry. Although such compounds are well documented in the literature rhodium(III) cyclometalates with azo function are spare. A typical example of this category viz. novel hexacoordinated orthometalated rhodium(III) thiolato complex trans-[Rh(C∧N∧S)Cl(PPh3)2] was synthesized from benzyl 2-(phenylazo)phenyl thioether and RhCl3·3H2O in the presence of excess PPh3 via in situ C(sp2)−H and C(sp3)−S bond scissions. This is the first example for a coordination compound of (phenylazo)thiolate ligand. The mechanism of formation of orthometalated azobenzene derivative was described to proceed via initial coordination of azo-nitrogen followed by electrophilic substitution at the pendant phenyl ring. PPh3 plays a crucial role in the C(sp3)−S cleavage process. Reductive cleavage by single electron transfer (SET) mechanism is likely to be operative for the C−S bond cleavage. Unlike analogous (phenylazo)phenolato compound the orthometalated thiolato complex exhibits a fully reversible oxidative wave at 0.82 V vs Ag/AgCl and this response is supposed to be primarily centered on the thiolato sulfur atom [2].
See also
- Chemical bonds of carbon with other elements in the periodic table:
CH He CLi CBe CB CC CN CO CF Ne CNa CMg CAl CSi CP CS CCl CAr CK CCa CSc CTi CV CCr CMn CFe CCo CNi CCu CZn CGa CGe CAs CSe CBr CKr CRb CSr CY CZr CNb CMo CTc CRu CRh CPd CAg CCd CIn CSn CSb CTe CI CXe CCs CBa CHf CTa CW CRe COs CIr CPt CAu CHg CTl CPb CBi CPo CAt Rn Fr Ra Rf Db Sg Bh Hs Mt Ds Rg Cn Uut Uuq Uup Uuh Uus Uuo ↓ CLa CCe CPr CNd CPm CSm CEu CGd CTb CDy CHo CEr CTm CYb CLu Ac Th Pa CU Np Pu Am Cm Bk Cf Es Fm Md No Lr Chemical bonds to carbon Core organic chemistry Many uses in chemistry Academic research, but no widespread use Bond unknown / not assessed References
- ^ Synthesis of Organometallic Compounds: A Practical Guide Sanshiro Komiya Ed. S. Komiya, M. Hurano 1997
- ^ K. Pramanik, U. Das, B. Adhikari, D. Chopra, H. Stoeckli-Evans (2008). "RhCl3-Assisted C-H and C-S Bond Scissions: Isomeric Self-Association of Organorhodium(III) Thiolato Complex. Synthesis, Structure, and Electrochemistry". Inorg. Chem. 47: 429–438. doi:10.1021/ic7016006. PMID 18161963.
Categories:- Organorhodium compounds
Wikimedia Foundation. 2010.