- Metallacycle
-
In organometallic chemistry, a metallacycle is a derivative of a carbocyclic compound wherein a metal has replaced at least one carbon center.[1][2] Metallacycles appear frequently as reactive intermediates in catalysis, e.g. olefin metathesis and alkyne trimerization. In organic synthesis, directed ortho metalation is widely used for the functionalization of arene rings via C-H activation. One main effect that metallic atom substitution on a cyclic carbon compound is distorting the geometry due to the large size of typical metals.
Contents
Nomenclature
Typically, metallacycles are cyclic compounds with two metal carbon bonds.[3]
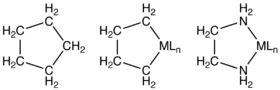 Structure of a carbocycle (cyclopentane), a metallacycle (a metallacyclopentane), and a metal chelated to ethylenediamine, a metal-containing ring that is not classified as a metallacycle.
Structure of a carbocycle (cyclopentane), a metallacycle (a metallacyclopentane), and a metal chelated to ethylenediamine, a metal-containing ring that is not classified as a metallacycle.
Many compounds containing metals in rings are known, for example chelate rings. Usually, such compounds are not classified as metallacycles, but the naming conventions are not rigidly followed. Within the area of coordination chemistry and supramolecular chemistry, examples include metallacrowns, metallacryptands, metallahelices, and molecular wheels.
Classes of metallacycles
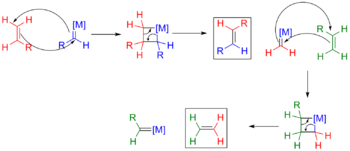
Metal-alkene complexes can be viewed as the smallest metallacycles, but they usually are not classified as metallacycles. In the Dewar-Chatt-Duncanson model, one resonance structure for the M(η2-alkene) center is the metallacyclopropane.
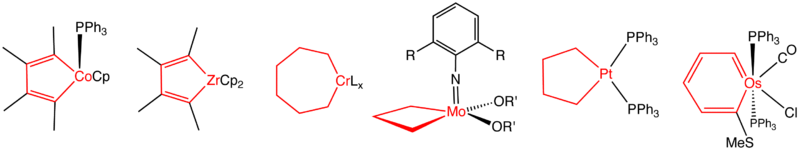 Representative metallacycles. From the left, a cobaltacyclopentadiene (a trapped intermediate from alkyne trimerization), zirconacyclopentadiene, chromacycloheptane (intermediate in trimerization of ethylene, L is unspecified), a molybdacyclobutane, a platinacyclopentane, and an osmabenzene.
Representative metallacycles. From the left, a cobaltacyclopentadiene (a trapped intermediate from alkyne trimerization), zirconacyclopentadiene, chromacycloheptane (intermediate in trimerization of ethylene, L is unspecified), a molybdacyclobutane, a platinacyclopentane, and an osmabenzene.
Metallacyclobutanes
The parent metallacyclobutane has the formula LnM(CH2)3 where L is a ligand attached to M. A stable example is (PPh3)2Pt(CH2)3.
Metallacyclobutane intermediates are involved in the alkene metathesis and in the oligomerization and dimerization of ethylene. In alkene metathesis, the Chauvin mechanism invokes the attack of an alkene at an electrophilic metal carbene catalyst.[4][5][6] This work helped to validate the Chauvin mechanism for olefin metathesis.
Metallacyclopentadienes
The parent metallacyclopentadiene has the formula LnM(CH)4. Most arise from the coupling of two alkynes at a low valent metal centers such as derivatives of Co(I) and Zr(II). Late metal derivatives (Co, Ni) are intermediates in the metal-catalysed trimerization of alkynes to arenes. Early metal derivatives, i.e. derivatives of Ti and Zr, are used stoichiometrically.[3] For example, the zirconacyclopentadiene Cp2ZrC4Me4 is a useful carrier for C4Me42-.[7]
Metallacyclopentanes
The parent metallacyclopentane has the formula LnM(CH2)4. Such compounds are intermediates in the metal catalysed dimerization, trimerization, and tetramerization of ethylene to give 1-butene, 1-hexene, and 1-octene, respectively.[8][9]
Metallabenzenes
The parent metallacyclobutane has the formula LnM(CH)5. Since the discovery of the structure of benzene,[10] many analogues of this iconic structure have been described. Examples include pyridine, phosphabenzene, arsabenzene, and pyrylium. Included in this group are the metallabenzenes.[11]
Metallabenzene complexes have been classified into three varieties; in such compounds the parent acyclic hydrocarbon ligand is viewed as the anion C5H5-. The 6 π electrons in the metallacycle conform to the Hückel (4n+2) theory.[12]
The first reported stable metallabenzene was the osmabenzene Os(C5H4S)CO(PPh3)2.[13][11] Characteristic of other metallaarenes, the Os-C bonds are about 0.6 Å longer than the C-C bonds (in benzene these are 1.39 Å), resulting in a distortion of the hexagonal ring. 1H NMR signals for the ring protons are downfield, consistent with aromatic "ring current." Osmabenzene and its derivatives can be regarded as an Os(II), d6 octahedral complex.
Isolated and characterized metallabenzenes have been also been reported with metals ruthenium,[14][15][16][17] iridium,[18][19] platinum [20][21][22] and rhenium [23]
Ortho-metallation
Metallacycles often arise by cyclization of arene-containing donor ligands, e.g. aryl phosphines and amines. An early example is the cyclization of IrCl(PPh3)3 to give the corresponding Ir(III) hydride containing a four-membered IrPCC ring.[24] Palladium(II) and platinum(II) have long been known to ortho-metallate aromatic ligands such as azobenzene, benzylamines, and 2-phenylpyridines.[25] These reactions are strongly influenced by substituent effects, including the Thorpe-Ingold Effect.[26] Ligands that lack aryl substituents will sometimes cyclometalate via activation of methyl groups, an early example being the internal oxidative addition of methylphosphine ligands.[27] Metallacycle formation interferes with intermolecular C-H activation processes. For this reason, specialized "pincer ligands" ligands have been developed that resist ortho-metallation.
References
- ^ Elschenbroich, C. ”Organometallics” (2006) Wiley-VCH: Weinheim. ISBN 978-3-29390-6
- ^ Jolly, William L. (1989). Modern Inorganic Chemistry (third ed.). McCraw-Hill. ISBN 0-07-032760-2.
- ^ a b Uwe Rosenthal, Vladimir V. Burlakov, Marc A. Bach and Torsten Beweries "Five-membered metallacycles of titanium and zirconium – attractive compounds for organometallic chemistry and catalysis" Chem. Soc. Rev. 2007, volume 36, 719-728. doi:10.1039/b605734a
- ^ "Press Release: The Nobel Prize in Chemistry 2005". http://nobelprize.org/nobel_prizes/chemistry/laureates/2005/press.html. Retrieved 3 December 2009.
- ^ Hérisson, J.-L.; Chauvin, Y. (1971). "Catalyse de transformation des oléfines par les complexes du tungstène. II. Télomérisation des oléfines cycliques en présence d'oléfines acycliques". Makromol. Chem. 141: 161. doi:10.1016/0022-328X(86)84064-5.
- ^ Schrock, R.R. (1986). "On the trail of metathesis catalysts". J. Organomet. Chem. 300: 249. doi:10.1016/0022-328X(86)84064-5.
- ^ Paul J. Fagan, William A. Nugent (1998), "1-Phenyl-2,3,4,5-Tetramethylphosphole", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV9P0653; Coll. Vol. 9: 653
- ^ John T. Dixon, Mike J. Green, Fiona M. Hess, David H. Morgan “Advances in selective ethylene trimerisation – a critical overview” Journal of Organometallic Chemistry 2004, Volume 689, Pages 3641-3668. doi:10.1016/j.jorganchem.2004.06.008
- ^ Feng Zheng, Akella Sivaramakrishna, John R. Moss “Thermal studies on metallacycloalkanes” Coordination Chemistry Reviews 2007, Volume 251, 2056-2071. doi:10.1016/j.ccr.2007.04.008
- ^ Kekulé, A. (1865). "Sur la constitution des substances aromatiques". Bull. Soc. Chim. 3: 98.
- ^ a b Bleeke, J.R. (2001). "Metallabenzenes". Chem. Rev. 101 (5): 1205. doi:10.1021/cr990337n. PMID 11710218.
- ^ Thorn, D.L.; Hoffmann, R. (1979). "Delocalization in Metallocycles". Nouv. J. Chim. 3 (1): 39.
- ^ Elliott, G.P.; Roper, W.R.; Waters, J.M. (1982). "Metallacyclohexatrienes or 'metallabenzenes.' Synthesis of osmabenzene derivatives and X-ray crystal structure of [Os(CSCHCHCHCH)(CO)(PPh3)2]". J. Chem. Soc., Chem. Commun. (14): 811. doi:10.1039/C39820000811.
- ^ Zhang, H.; Xia, H.; He, G.; Wen, T.; Gong, L.; Jia, G. (2006). "Synthesis and characterization of stable ruthenabenzenes". Angewandte Chemie (International ed. in English) 45 (18): 2920–2923. doi:10.1002/anie.200600055. PMID 16566052.
- ^ Zhang, H.; Feng, L.; Gong, L.; Wu, L.; He, G.; Wen, T.; Yang, F.; Xia, H. (2007). "Synthesis and Characterization of Stable Ruthenabenzenes Starting from HC⋮CCH(OH)C⋮CH". Organometallics 26 (10): 2705. doi:10.1021/om070195k.
- ^ Wu, L.; Feng, L.; Zhang, H.; Liu, Q.; He, X.; Yang, F.; Xia, H. (2008). "Synthesis and characterization of a novel dialdehyde and cyclic anhydride". The Journal of organic chemistry 73 (7): 2883–2885. doi:10.1021/jo800052u. PMID 18336045.
- ^ Clark, G. R.; O’neale, T. R.; Roper, W. R.; Tonei, D. M.; Wright, L. J. (2009). "Stable Cationic and Neutral Ruthenabenzenes". Organometallics 28 (2): 567. doi:10.1021/om800857k.
- ^ Bleeke, J. R.; Xie, Y. F.; Peng, W. J.; Chiang, M. (1989). "Metallabenzene: synthesis, structure, and spectroscopy of a 1-irida-3,5-dimethylbenzene complex". Journal of the American Chemical Society 111 (11): 4118. doi:10.1021/ja00193a064.
- ^ Bleeke, J. R.; Xie, Y. F.; Bass, L.; Chiang, M. Y. (1991). "Metallacyclohexadiene and metallabenzene chemistry. 5. Chemical reactivity of metallabenzene". Journal of the American Chemical Society 113 (12): 4703. doi:10.1021/ja00012a061.
- ^ Jacob, V.; Weakley, T. J. R.; Haley, M. M. (2002). "Metallabenzenes and Valence Isomers. Synthesis and Characterization of a Platinabenzene". Angewandte Chemie International Edition 41 (18): 3470. doi:10.1002/1521-3773(20020916)41:18<3470::AID-ANIE3470>3.0.CO;2-4.
- ^ Landorf, C. W.; Jacob, V.; Weakley, T. J. R.; Haley, M. M. (2004). "Rational Synthesis of Platinabenzenes†". Organometallics 23 (6): 1174. doi:10.1021/om034371a.
- ^ Jacob, V.; Landorf, C. W.; Zakharov, L. N.; Weakley, T. J. R.; Haley, M. M. (2009). "Platinabenzenes: Synthesis, Properties, and Reactivity Studies of a Rare Class of Metalla-aromatics†". Organometallics 28 (17): 5183. doi:10.1021/om900439z.
- ^ Poon, K. C.; Liu, L.; Guo, T.; Li, J.; Sung, H. H. Y.; Williams, I. D.; Lin, Z.; Jia, G. (2010). "Synthesis and Characterization of Rhenabenzenes". Angewandte Chemie International Edition 49 (15): 2759–2762. doi:10.1002/anie.200907014. PMID 20229549.
- ^ M. A. Bennett, D. L. Milner "Chlorotris(triphenylphosphine)iridium(I) and related complexes. Oxidative addition reactions and hydrogen abstraction from the coordinated ligand" J. Am. Chem. Soc. 1969, 91, 6983-6994.doi:10.1021/ja01053a016.
- ^ A. C. Cope, R. W. Siekman "Formation of Covalent Bonds from Platinum or Palladium to Carbon by Direct Substitution" J. Am. Chem. Soc. 1965, volume 87, 3272-3273. doi:10.1021/ja01092a063
- ^ Shaw, B. L., "Formation of large rings, internal metalation reactions, and internal entropy effects", J. Am. Chem. Soc. 1975, volume 97, 3856-3857. doi:10.1021/ja00846a072.
- ^ J. Chatt and J. M. Davidson "The tautomerism of arene and ditertiary phosphine complexes of ruthenium(0), and the preparation of new types of hydrido-complexes of ruthenium(II)" J. Chem. Soc. 1965, 843. doi:10.1039/JR9650000843
Categories:
Wikimedia Foundation. 2010.