- Organoruthenium chemistry
-
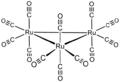
Organoruthenium chemistry is the chemistry of organometallic compounds containing a carbon to ruthenium chemical bond.[1] The interest is mostly academic although several organoruthenium catalysts are of commercial interest. The chemistry has many similarities with organoiron chemistry, as iron is directly above ruthenium in group 8 of the periodic table. The most important reagents for the introduction of ruthenium are ruthenium(III) chloride and triruthenium dodecacarbonyl. The related compound Ru(CO)5 is less attractive for this purpose because it decomposes:
- Ru3(CO)12 + 3 CO
 3 Ru(CO)5
3 Ru(CO)5
In its organometallic compounds, ruthenium is known to adopt oxidation states from -2 ([Ru(CO)4]2-) to +6 ([RuN(Me)4]-). Most common are those in the 2+ oxidation state, as illustrated below.
Contents
Ligands
The most important ligands for ruthenium are:
- carbon monoxide as in metal carbonyls
- phosphines. BINAP is an asymmetric ligand for many ruthenium compounds in asymmetric synthesis [2] [3] [4] [5].
- cyclopentadienyl ligands.
- various arenes and dienes
- metal carbenes, notably Grubbs' catalyst, an import catalyst in olefin metathesis.
- halides. chlorine ligands are easily replaced by alkyl, aryl or hydride ligands
- hydrogen. Transition metal hydrides are important in C-H bond activation.
Cyclopentadienyl ligands
The parent compound ruthenocene is unreactive because it is coordinatively saturated and contains no reactive groups. Shvo's catalyst ({[Ph4(η5-C4CO)]2H]}Ru2(CO)4(μ-H)) is also coordinatively saturated, but features reactive OH and RuH groups that enable it to function in transfer hydrogenation.[6]. It is used in hydrogenation of aldehydes, ketones, via transfer hydrogenation, in disproportionation of aldehydes to esters and in the isomerization of allylic alcohols.
Chloro(cyclopentadienyl)bis(triphenylphosphine)ruthenium features a reactive chloro group, which is readily substituted by organic substrates.
Arene and alkene ligands
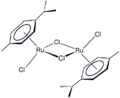
One example of an Ru-arene complex is (cymene)ruthenium dichloride dimer, which is the precursor to a versatile catalyst for transfer hydrogenation.[7] Acenaphthylene forms a useful catalyst derived from triruthenium dodecacarbonyl.[8] The hapticity of the hexamethylbenzene ligand in Ru(C6Me6)2 depends on the oxidation state of the metal centre (methyl groups omitted for clarity):[9]
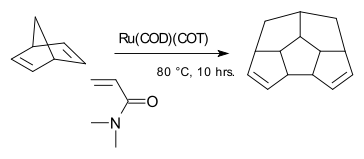
The compound Ru(COD)(COT) is capable of dimerizing norbornadiene:
Organoosmium
In the same group 8 elements osmium resembles ruthenium in its complexes. Because Os is more expensive than Ru, the chemistry is less developed and has fewer applications. Of course the cost of the catalyst is offset of turnover numbers are high. Thus, Osmium tetroxide is an important oxidizing agent in organic chemistry especially in the conversion of alkenes to 1,2-diols.
The 5d-orbitals in Os are higher in energy that the 4d-orbitals in Ru. Thus, π backbonding to alkenes and CO is stronger for Os compounds, which leads to more stable organic derivatives. This effect is illustrated by the stability of the alkene derivatives of the type [Os(NH3)5(alkene)]2+ or [Os(NH3)5(arene)]2+ as in the example below.
Important compounds, at least for academic studies, are the carbonyls such as triosmium dodecacarbonyl and decacarbonyldihydridotriosmium. The phosphine complexes are analogous to those or ruthenium, but hydride derivatives tend to be more stable, e.g. OsHCl(CO)(PPh3)3.
See also
- Chemical bonds of carbon with other elements in the periodic table:
CH He CLi CBe CB CC CN CO CF Ne CNa CMg CAl CSi CP CS CCl CAr CK CCa CSc CTi CV CCr CMn CFe CCo CNi CCu CZn CGa CGe CAs CSe CBr CKr CRb CSr CY CZr CNb CMo CTc CRu CRh CPd CAg CCd CIn CSn CSb CTe CI CXe CCs CBa CHf CTa CW CRe COs CIr CPt CAu CHg CTl CPb CBi CPo CAt Rn Fr Ra Rf Db Sg Bh Hs Mt Ds Rg Cn Uut Uuq Uup Uuh Uus Uuo ↓ CLa CCe CPr CNd CPm CSm CEu CGd CTb CDy CHo CEr CTm CYb CLu Ac Th Pa CU Np Pu Am Cm Bk Cf Es Fm Md No Lr Chemical bonds to carbon Core organic chemistry Many uses in chemistry Academic research, but no widespread use Bond unknown / not assessed References
- ^ Synthesis of Organometallic Compounds: A Practical Guide Sanshiro Komiya Ed. S. Komiya, M. Hurano 1997
- ^ Example: Organic Syntheses, Coll. Vol. 10, p.276 (2004); Vol. 77, p.1 (2000). Link
- ^ Example: Organic Syntheses, Organic Syntheses, Coll. Vol. 9, p.589 (1998); Vol. 71, p.1 (1993). Link
- ^ Example: Organic Syntheses, Organic Syntheses, Coll. Vol. 9, p.169 (1998); Vol. 72, p.74 (1995). Link
- ^ Example: Organic Syntheses, Vol. 81, p.178 (2005). Link
- ^ Conley, B.; Pennington-Boggio, M.; Boz, E.; Williams, T. (2010). "Discovery, Applications, and Catalytic Mechanisms of Shvo's Catalyst". Chemical reviews 110 (4): 2294–2312. doi:10.1021/cr9003133. PMID 20095576.
- ^ Organic Syntheses, Organic Syntheses, Vol. 82, p.10 (2005).Link
- ^ Example: Organic Syntheses, Organic Syntheses, Vol. 82, p.188 (2005). Link
- ^ Huttner, Gottfried; Lange, Siegfried; Fischer, Ernst O. (1971). "Molecular Structure of Bis(Hexamethylbenzene)-Ruthenium(0)". Angewandte Chemie, International Edition in English 10 (8): 556–557. doi:10.1002/anie.197105561.
Categories:- Organoruthenium compounds
- Ru3(CO)12 + 3 CO
Wikimedia Foundation. 2010.