- Chloro(cyclopentadienyl)bis(triphenylphosphine)ruthenium
-
CpRuCl(PPh3)2 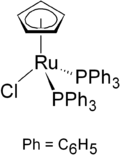
Identifiers CAS number 32993-05-8 Properties Molecular formula C41H35ClP2Ru Molar mass 726.19 g/mol Appearance Orange solid Melting point 135°C (408 K)
Solubility in water Insoluble  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Chloro(cyclopentadienyl)bis(triphenylphosphine)ruthenium is the organoruthenium half-sandwich compound with formula RuCl(PPh3)2(C5H5). It as an air-stable orange crystalline solid that is used in a variety of organometallic synthetic and catalytic transformations.
Contents
Structure and Properties
The compound has idealized Cs symmetry. It is soluble in chloroform, dichloromethane, and acetone.
Preparation
Chloro(cyclopentadienyl)bis(triphenylphosphine)ruthenium was first reported in 1969 when it was prepared by reacting dichlorotris(triphenylphosphine)ruthenium(II) with cyclopentadiene.[1]
- RuCl2(PPh3)3 + C5H6 → RuCl(PPh3)3(C5H5) + HCl
It is easily prepared from ruthenium(III) chloride, triphenylphosphine, and cyclopentadiene in ethanol.[2]
Reactions
Chloro(cyclopentadienyl)bis(triphenylphosphine)ruthenium(II) undergoes a variety of substitution reactions often by involving substitution of the chloride. With phenylacetylene it gives the phenyl vinylidene complex.
- (C5H5)(PPh3)2RuCl + HC2Ph + NH4[PF6] → [Ru(C:CHPh)(PPh3)2(C5H5)][PF6] + NH4Cl
Displacement of PPh3 by carbon monoxide affords a chiral compound.[3]
- (C5H5)(PPh3)2RuCl + CO → (C5H5)(PPh3)(CO)RuCl + PPh3
The compound can also be converted into the hydride.[4]
- (C5H5)(PPh3)2RuCl + NaOMe → (C5H5)(PPh3)2RuH + NaCl + CH2O
Applications
Chloro(cyclopentadienyl)bis(triphenylphosphine)ruthenium(II) serves as a catalyst for a variety of reactions. For example, in the presence of NH4PF6 it catalyzes the isomerisation of allylic alcohols to the corresponding saturated carbonyls.[5]
References
- ^ Gilbert, J.; Wilkinson, G. (1969). "New Complexes of Ruthenium(II) with Triphenylphosphine and other Ligands". J. Chem. Soc.: 1749. doi:10.1039/J19690001749.
- ^ Bruce, M., Hamiester, C., Swincer, A., Wallis, R., and Ittel, S. (1982). “Some -Cyclopentadienylruthenium(II) Complexes Containing Triphenylphosphine.” Inorganic Syntheses (1982). 78-82. DOI: 10.1002/9780470132524
- ^ Blackmore, T., Bruce, M., and Stone, F. (1971). "New Cyclopentadienyltuthenium Complexes". J. Chem. Soc., A: 2376–2382. doi:10.1039/J19710002376.
- ^ Wilczewski, T., Bochenska, M., and Biernat, J. (1981). "Cyclobentadienyl-Ruthenium Complexes". J. Organomet. Chem. 215: 87. doi:10.1016/S0022-328X(00)84619-7.
- ^ Murahashi, Shun-Ichi. “Ruthenium in Organic Synthesis” (2006) Wiley-VCH: Weinheim. ISBN 978-3-527-30692-3
Ruthenium compounds RuB2 · RuO2 · RuCl3 · RuO4 · N(C3H7)4RuO4 · C72H42N6Na4O18RuS6 · Ru3(CO)12 · (Ru(bipy)3)Cl2 · C62H42O6Ru2 · C54H45Cl2P3Ru · C8H24Cl2O4RuS4 · C56H45O2P3Ru · · C20H28Cl4Ru2 · C41H35ClP2Ru · (C5H5)2Ru
Categories:- Ruthenium compounds
Wikimedia Foundation. 2010.
