- Carborane
-
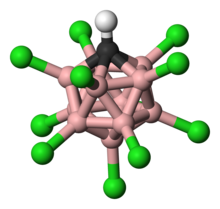
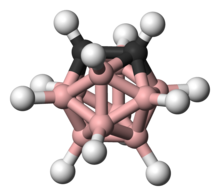 Ball-and-stick model of o-carborane
Ball-and-stick model of o-carborane
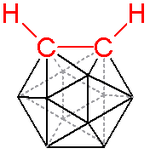
A carborane is a cluster composed of boron and carbon atoms. Like many of the related boranes, these clusters are polyhedra and are similarly classified as closo-, nido-, arachno-, hypho-, etc. based on whether they represent a complete (closo-) polyhedron, or a polyhedron that is missing one (nido-), two (arachno-), or more vertices.
Interesting examples of carboranes are the extremely stable icosahedral closo-carboranes.[1]
A prominent example is the charge-neutral C2B10H12 or o-carborane with the prefix o derived from ortho, which has been explored for use in a wide range of applications from heat-resistant polymers to medical applications. The electronic structure of these compounds is best described by Wade-Mingos rules for cluster molecules. At 420 °C o-carborane converts to the meta isomer. In comparison, benzene requires a >1000 °C to induce skeletal rearrangement. Like arenes, carboranes also undergo electrophilic aromatic substitution.
Another important carborane is the negatively charged CHB11H12−, which has been used to make solid superacids.
Contents
Dicarbadodecaborane
The most heavily studied carborane is C2B10H12, m. p. 320 °C. It is often prepared from the reaction of acetylene with decaborane. A variation on this method entails the use of dimethyl acetylenedicarboxylate to give C2B10H10(CO2C H3)2, which can be degraded to the C2B10H12.[2]
History
The 1,2-closo-dicarbadodecaboranes (usually simply called carboranes), were reported simultaneously by groups at Olin Corporation and the Reaction Motors Division of Thiokol Chemical Corporation working under the U.S. Air Force and published in 1963.[3][4][5][6][7][8][9][10][11] Heretofore, decaborane derivatives were thought to be thermally unstable and reactive with air and water. These groups demonstrated the unprecedented stability of the 1,2-closo-dodecaborane group, presented a general synthesis, described the transformation of substituents without destroying the carborane cluster, and demonstrated the ortho to meta isomerization.
Dicarbollide
Numerous studies have been made on derivatives of the so-called dicarbollide anion, [B9C2H11]2−. The first metal dicarbollide complex was discovered by M. Frederick Hawthorne and co-workers in 1965.[12] This anion forms sandwich compounds, referred to as bis(dicarbollides), with many metal ions and some exist in otherwise unusual oxidation states. The dianion is a nido cluster prepared by degradation of the parent dicarborane:[13]
- B10C2H12 + 3 CH3OH + KOH → KB9C2H12 + B(OCH3)3 + H2O + H2
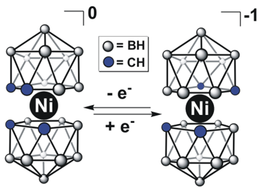
Bis(dicarbollides) often exhibit properties very different from their metallocene surrogates. For example, Ni-based bis(dicarbollide) cluster can be observed for the rare Ni(IV) oxidation state of nickel. Some notable examples of potential applications of these complexes include catalysis,[14] ion-exchange materials for radioactive waste management, biologically active protease inhibitors, and chemically inert redox shuttles for dye-sensitized solar cells (DSSCs).[15]
Carborynes
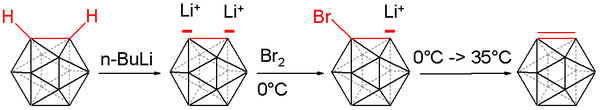
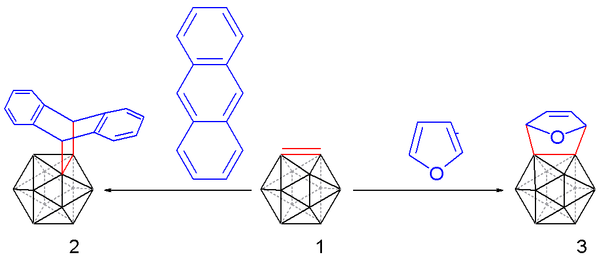
Carboryne, or 1,2-dehydro-o-carborane, is an unstable derivative of ortho-carborane with the formula B10C2H10. The hydrogen atoms on the C2 unit in the parent o-carborane are missing. The compound resembles and is isolobal with benzyne.[16][17][18] A carboryne compound was first generated in 1990 starting from o-carborane. The hydrogen atoms connected to carbon are removed by n-butyllithium in tetrahydrofuran and the resulting lithium dianion is reacted with bromine at 0 °C to form the bromo monoanion.
Heating the reaction mixture to 35 °C releases carboryne, which can subsequently be trapped with suitable dienes:
such as anthracene (to afford a trypticene-like molecule) and furan in 10 to 25% chemical yield.
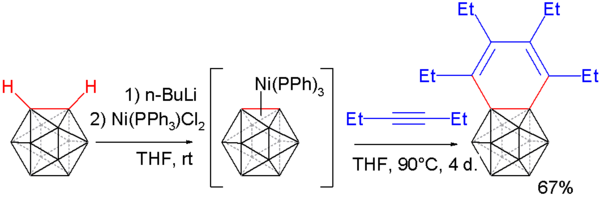
Carborynes react with alkynes to benzocarboranes [19][20] in an adaptation of the above described procedure. O-carborane is deprotonated with n-butyllithium as before and then reacted with dichloro-di(triphenylphosphino) nickel to a nickel coordinated carboryne. This compound reacts with 3-hexyne in an alkyne trimerization to the benzocarborane.
Single crystal X-ray diffraction analysis of this compound shows considerable bond length alternation in the benzene ring (164.8 pm to 133.8 pm) ruling out aromaticity.
Applications
Carboranes have been used as a source of boron in boron neutron capture therapy.[21] They have also been used in structural studies in crystallography.[22]
Carboranes have been used to make solid superacids. Solid superacid catalysts alleviate the need to dispose of spent acids, thus providing a significant environmental advantage over dissolved acids.[23] The carborane superacid H(CHB11Cl11)[24] is one million times stronger than sulfuric acid.[25][26] The reason for this high acidity is that the acid anion CHB11Cl11− is very stable and substituted with electronegative substituents. H(CHB11Cl11) is the only acid known to protonate C60 fullerene without decomposing it.[27][28] Additionally, it is the only known anion capable of forming a stable, isolable salt with protonated benzene, C6H7+. In coordination chemistry carboranes can be used as unique bulky ligand scaffolds. It was recently demonstrated, that the same carboranyl moiety can act either as strongly electron-withdrawing or electron-donating substituent, depending on the positional attachment of the cluster to heteroatom.[29]
References
- ^ Eluvathingal D. Jemmis (1982). "Overlap control and stability of polyhedral molecules. Closo-Carboranes". J. Am. Chem. Soc. 104 (25): 7017–7020. doi:10.1021/ja00389a021.
- ^ Kutal, C. R.; Owen, D. A.; Todd, L. J. (1968). "Closo-1,2-dicarbadodecaborane(12)". Inorganic Syntheses. Inorganic Syntheses 11: 19–23. doi:10.1002/9780470132425.ch5. ISBN 9780470132425.
- ^ T. L. Heying, J. W. Ager, S. L. Clark, D. J. Mangold, H. L. Goldstein, M. Hillman, R. J. Polak, and J. W. Szymanski (1963). "A New Series of Organoboranes. I. Carboranes from the Reaction of Decaborane with Acetylenic Compounds". Inorganic Chemistry 2 (6): 1089–1092. doi:10.1021/ic50010a002.
- ^ H. Schroeder, T. L. Heying, J. R. Reiner (1963). "A New Series of Organoboranes. II. The Chlorination of 1,2-Dicarbaclosododecaborane(12)" Inorganic Chemistry". Inorganic Chemistry 2 (6): 1092–1096. doi:10.1021/ic50010a003.
- ^ T. L. Heying, J. W. Ager, S. L. Clark, R. P. Alexander, S. Papetti, J. A. Reid, S. I. Trotz (1963). "A New Series of Organoboranes. III. Some Reactions of 1,2-Dicarbaclosododecaborane(12) and its Derivatives". Inorganic Chemistry 2 (6): 1097–1105. doi:10.1021/ic50010a004.
- ^ S. Papetti, T. L. Heying (1963). "A New Series of Organoboranes. IV. The Participation of the 1,2-Dicarbaclosododecaborane(12) Nucleus in Some Novel Heteratomic Ring Systems". Inorg. Chem. 2 (6): 1105–1107. doi:10.1021/ic50010a005.
- ^ M. M. Fein, J. Bobinski, N. Mayes, N. Schwartz, M. S. Cohen (1963). "Carboranes. I. The Preparation and Chemistry of 1-Isopropenylcarborane and its Derivatives (a New Family of Stable Closoboranes)". Inorg. Chem. 2 (6): 1111–1115. doi:10.1021/ic50010a007.
- ^ M. M. Fein, D. Grafstein, J. E. Paustian, J. Bobinski, B. M. Lichstein, N. Mayes, N. N. Schwartz, M. S. Cohen (1963). "Carboranes. II. The Preparation of 1- and 1,2-Substituted Carboranes". Inorg. Chem. 2 (6): 1115–1119. doi:10.1021/ic50010a008.
- ^ D. Grafstein, J. Bobinski, J. Dvorak, H. Smith, N. Schwartz, M. S. Cohen, M. M. Fein (1963). "Carboranes. III. Reactions of the Carboranes". Inorg. Chem. 2 (6): 1120–1125. doi:10.1021/ic50010a009.
- ^ D. Grafstein, J. Bobinski, J. Dvorak, J. E. Paustian, H. F. Smith, S. Karlan, C. Vogel, M. M. Fein (1963). "Carboranes. IV. Chemistry of Bis-(1-carboranylalkyl) Ethers". Inorg. Chem. 2 (6): 1125–1128. doi:10.1021/ic50010a010..
- ^ D. Grafstein, J. Dvorak (1963). "Neocarboranes, a New Family of Stable Organoboranes Isomeric with the Carboranes". Inorg. Chem. 2 (6): 1128–1133. doi:10.1021/ic50010a011.
- ^ M. F. Hawthorne, D. C. Young, P. A. Wegner, J. Am. Chem. Soc. 87, 1965, 1818-1819 Carbametallic Boron Hydride Derivatives. I. Apparent Analogs of Ferrocene and Ferricinium Ion
- ^ Plešek, J.; Heřmánek, S.; Štíbr, B. Inorganic Syntheses, 1983, volume 22, pages 231-124.
- ^ Crowther, D. J., Baenziger, N. C., Jordan, R. F. Group 4 metal dicarbollide chemistry. Synthesis, structures and reactivity of electrophilic alkyl complexes (Cp*)(C2B9H11)M(R), M = Hf, Zr. J. Am. Chem. Soc. 113, 1455-1457 (1991).
- ^ Alexander M. Spokoyny, Tina C. Li, Charles W. Machan, Omar K. Farha, Chunxing She, Charlotte L. Stern, Tobin J. Marks, Joseph T. Hupp, Chad A. Mirkin (2010). "Electronic Tuning of Nickel-Based Bis(dicarbollide) Redox Shuttles in Dye-Sensitized Solar Cells". Angewandte Chemie, International Edition 49 (31): 5339 –5343. doi:10.1002/anie.201002181.
- ^ Henry L. Gingrich, Tirthankar Ghosh, Qiurong Huang, and Maitland Jones (1990). "1,2-Dehydro-o-carborane". J. Am. Chem. Soc. 112 (10): 4082–4083. doi:10.1021/ja00166a080.
- ^ E. D. Jemmis and B. Kiran (1997). "Structure and Bonding in B10X2H10 (X = C and Si). The Kinky Surface of 1,2-Dehydro-o-Disilaborane". J. Am. Chem. Soc. 119 (19): 4076–4077. doi:10.1021/ja964385q.
- ^ B. Kiran, A. Anoop, E. D. Jemmis (2002). "Control of Stability through Overlap Matching: closo-Carboranes and closo-Silaboranes". J. Am. Chem. Soc. 124 (16): 4402–4407. doi:10.1021/ja016843n.
- ^ Liang Deng, Hoi-Shan Chan, and Zuowei Xie (2006). "Nickel-Mediated Regioselective [2 + 2 + 2] Cycloaddition of Carboryne with Alkynes". J. Am. Chem. Soc. 128 (24): 7728–7729. doi:10.1021/ja061605j. PMID 16771473.
- ^ Eluvathingal D Jemmis and Anakuthil Anoop (2004). "Theoretical Study of the Insertion Reactions of Benzyne- and Carboryne- Ni Complexes". MHPCC Application Briefs: 51. http://www.mhpcc.hpc.mil/research/appbriefs/2004/2004MHPCCAppBriefs.pdf.
- ^ Albert H. Soloway; Werner Tjarks; Beverly A. Barnum; Feng-Guang Rong; Rolf F. Barth; Iwona M. Codogni; J. Gerald Wilson (1998). "The Chemistry of Neutron Capture Therapy". Chem. Rev. 98 (4): 1515–1562. doi:10.1021/cr941195u.
- ^ D.P. Zhang; J.M. Dou; D.C. Li; D.Q. Wang (2007). "Di-μ-chlorido-bis{[1,2-bis(diphenylphosphino)-1,2-dicarba-closo-dodecaborane-2
κP,P']silver(I)} dichloromethane disolvate". Acta Crystallographica E63: m1086-m1088. doi:10.1107/S1600536807007088. - ^ George Andrew Olah (2001). A life of magic chemistry: autobiographical reflections of a nobel prize winner. John Wiley and Sons. p. 105. ISBN 0471157430. http://books.google.com/?id=DjAboQVLjOsC&printsec=frontcover&q.
- ^ Note that the image the acidic proton is not the one bonded to the carborane but that it is the counterion not displayed
- ^ George A. Olah, et. al. Superacid Chemistry, 2nd ed., Wiley, p. 41.
- ^ That is, the concentration of H+ in a solution of the carborane superacid is a million times higher than in a solution of sulfuric acid.
- ^ Mark Juhasz, Stephan Hoffmann, Evgenii Stoyanov, Kee-Chan Kim, Christopher A. Reed (2004). "The Strongest Isolable Acid". Angewandte Chemie International Edition 43 (40): 5352–5355. doi:10.1002/anie.200460005. PMID 15468064.
- ^ Christopher A. Reed (2005). "Carborane acids. New "strong yet gentle" acids for organic and inorganic chemistry" (Full article (reprint)). Chem. Commun. 2005 (13): 1669–1677. doi:10.1039/b415425h. PMID 15791295. http://www.reedgrouplab.ucr.edu/publications/Chem%20Comm%202005%201669.pdf.
- ^ Alexander M. Spokoyny, Charles W. Machan, Daniel J. Clingerman, Mari S. Rosen, Michael J. Wiester, Robert D. Kennedy, Charlotte L. Stern, Amy A. Sarjeant, Chad A. Mirkin (2011). "A coordination chemistry dichotomy for icosahedral carborane-based ligands". Nature Chemistry 3 (8): 590–596. doi:10.1038/nchem.1088. PMID 21778977.
External links
Categories:- Organoboron compounds
- Cluster chemistry
- Superacids
Wikimedia Foundation. 2010.