- Dimethylformamide
-
N,N-Dimethylformamide 
 N,N-DimethylmethanamideOther namesDMF; Dimethylformamide; N,N-Dimethylformamide; DMFA
N,N-DimethylmethanamideOther namesDMF; Dimethylformamide; N,N-Dimethylformamide; DMFAIdentifiers CAS number 68-12-2 
PubChem 6228 ChemSpider 5993 
UNII 8696NH0Y2X 
DrugBank DB01844 KEGG C03134 
ChEBI CHEBI:17741 
ChEMBL CHEMBL268291 
RTECS number LQ2100000 Jmol-3D images Image 1 - O=CN(C)C
Properties Molecular formula C3H7NO Molar mass 73.09 g/mol Appearance Clear liquid Density 0.944 g/cm3, liquid Melting point -61 °C, 212 K, -78 °F
Boiling point 153 °C, 426 K, 307 °F
Solubility in water Miscible Vapor pressure 0.3 kPa (@ 20°C) Refractive index (nD) 1.4305 (20 °C), εr = 36.71 (25°C) Viscosity 0.92 cP at 20 °C Structure Dipole moment 3.86 D (25 °C) Hazards MSDS MSDS R-phrases R61 R20/21 R36 S-phrases S53 S45 Main hazards flammable NFPA 704 Flash point 58 °C Threshold Limit Value 10 ppm[1] (TWA) Related compounds Related amides Acetamide,
Formamide,
hexamethylphosphoramideRelated compounds Dimethyl sulfoxide,
acetonitrile,
N-Methylformamide (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Dimethylformamide is an organic compound with the formula (CH3)2NC(O)H. Commonly abbreviated DMF (though this acronym is sometimes used for dimethylfuran), this colourless liquid is miscible with water and the majority of organic liquids. DMF is a common solvent for chemical reactions. Pure dimethylformamide is odorless whereas technical grade or degraded dimethylformamide often has a fishy smell due to impurity of dimethylamine. Its name is derived from the fact that it is a derivative of formamide, the amide of formic acid.
Dimethylformamide is a polar (hydrophilic) aprotic solvent with a high boiling point. It facilitates reactions that follow polar mechanisms, such as SN2 reactions. Dimethylformamide can be synthesized from methyl formate and dimethylamine or by reaction of dimethylamine with carbon monoxide.[2] Dimethylformamide is not stable in the presence of strong bases like sodium hydroxide or strong acids such as hydrochloric acid or sulfuric acid and is hydrolyzed back into formic acid and dimethylamine, especially at elevated temperatures.
Contents
Structure and properties
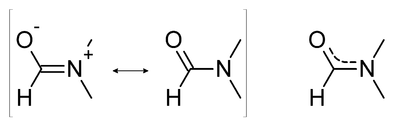
Due to the contribution of the two possible resonance structures of an amide, the bond order of the carbonyl C=O bond is reduced, while that of the carbon-nitrogen bond is increased. Thus the infrared spectrum of DMF shows a lower C=O stretching frequency at 1675 cm−1[3] than an unsubstituted C=O bond. Also, because of the partial double bond character, the rotation about the C-N axis is slow at room temperature, making the two methyl groups inequivalent on the NMR time scale, giving rise to two singlets of 3 protons each at δ 2.97 and 2.88,[3] instead of one singlet of 6 protons in the proton NMR spectrum.
N,N-Dimethylformamide (DMF) is miscible with water in all proportions.[4] The vapour pressure at 20°C is 3.5hPa.[5] A Henry's law constant of 7.47×10−5 hPa·m3/mol can be deduced from an experimentally determined equilibrium constant at 25 °C.[6] The partition coefficient logPOW is measured to –0.85.[7] Since the density of DMF (0.95 g/cm3 at 20 °C[4]) is very similar to that of water, significant flotation or stratification in surface waters in case of accidental losses is not expected.
Production
Dimethyl formamide is produced either via catalyzed reaction of dimethylamine and carbon monoxide in methanol or via the reaction of methyl formate with dimethylamine.[2] It may also be prepared on a laboratory scale by reacting dimethylamine with formic acid.[citation needed]
Applications
The primary use of dimethylformamide is as a solvent with low evaporation rate. DMF is used in the production of acrylic fibers and plastics. It is also used as a solvent in peptide coupling for pharmaceuticals, in the development and production of pesticides, and in the manufacture of adhesives, synthetic leathers, fibers, films, and surface coatings.[8]
- It is used as a reagent in the Bouveault aldehyde synthesis and in the Vilsmeier-Haack reaction, another useful method of forming aldehydes.
- It is also a common catalyst used in the synthesis of acyl halides, in particular the synthesis of acyl chlorides from carboxylic acids using oxalyl or thionyl chloride.[9]
- DMF penetrates most plastics and makes them swell. This property makes it very suitable for solid phase peptide synthesis. It also frequently occurs as a component of paint strippers for this purpose.
- DMF is very effective at separating and suspending carbon nanotubes, and is recommended by the NIST for use in near infrared spectroscopy of such.[10]
- DMF can be utilized as a standard in proton NMR allowing for a quantitative determination of an unknown chemical.
- DMF is used as a solvent to recover olefins such as 1,3-butadiene via extractive distillation.
- It is also used in the manufacturing of solvent dyes as an important raw material. It is consumed during reaction.
- Pure acetylene gas cannot be compressed and stored without the danger of explosion. Industrial acetylene gas is, therefore, dissolved in dimethylformamide and stored in metal cylinders or bottles. The casing is also filled with agamassan, which renders it safe to transport and use.
Safety
Reactions including the use of sodium hydride in DMF as a solvent are somewhat hazardous; exothermic decompositions have been reported at temperatures as low as 26 °C. On a laboratory scale any thermal runaway is (usually) quickly noticed and brought under control with an ice bath and this remains a popular combination of reagents. On a pilot plant scale, on the other hand, several accidents have been reported.[11]
Toxicity
DMF has been linked to cancer in humans, and it is thought to cause birth defects [12]. In some sectors of industry women are banned from working with DMF. For many reactions, it can be replaced with dimethyl sulfoxide. Most manufacturers of DMF list (Life) or (Chronic) as a health hazard in their MSDS since DMF is not readily disposed of by the body. According to IARC, DMF is a possible carcinogen, although EPA does not consider it a cancer risk.
References
- ^ ScienceLab.com MSDS
- ^ a b Klaus Weissermel, Hans-Jürgen Arpe. Industrial Organic Chemistry: Important Raw Materials and Intermediates. Wiley-VCH. pp. 45–46. ISBN 3527305785.
- ^ a b Spectral Database for Organic Compounds, Dimethylformamide, accessed 27 Jan 2007.
- ^ a b Bipp, H. and Kieczka, H. (1989). Ullmann’s Encyclopedia of Industrial Chemistry. A12 (5 ed.). Weinheim: VCH Verlagsgesellschaft. pp. 1–12.
- ^ IPCS (International Programme on Chemical Safety) (1991). Environmental Health Criteria 114 “Dimethylformamide” United Nations Environment Programme, International Labour Organisation, World Health Organization; 1-124.
- ^ Taft, R.W. et al. (1985). "The molecular properties governing solubilities of organic nonelectrolytes in water". Nature 313 (6001): 384–386. doi:10.1038/313384a0.
- ^ (BASF AG, department of analytical, unpublished data, J-No. 124659/08, 27.11.1987)
- ^ Redlich, C; Beckett, W. S.; Sparer, J.; Barwick, K. W.; Riely, C. A.; Miller, H.; Sigal, S. L.; Shalat, S. L. et al. (1988). "Liver disease associated with occupational exposure to the solvent dimethylformamide". Ann. Intern. Med. 108 (5): 680–686. PMID 3358569.
- ^ Clayden, Jonathan (2001). Organic chemistry. Oxford: Oxford University Press. pp. 276–296. ISBN 0-19-850346-6.
- ^ Haddon, Robert; Itkis, Mikhail (March, 2008) (PDF). Measurement Issues in Single Wall Carbon Nanotubes. NIST. pp. 20. http://www.nist.gov/public_affairs/practiceguides/NIST%20SP960-19.pdf. Retrieved 2008-08-15.
- ^ UK Chemical Reaction Hazards Forum and references cited therein
- ^ Hazardous substance fact sheet for Dimethylformamide
External links
- International Chemical Safety Card 0457
- NIOSH Pocket Guide to Chemical Hazards 0226
- Dimethylformamide usage on Organic Syntheses
- Concise International Chemical Assessment Document 31: N,N-Dimethylformamide
- Material Safety Data Sheet for DMF
- DMF Chronic Toxicity Summary (PDF)
- Dimethylformamide Technical Specs from BASF(pdf)
- DMF GHS MSDS from NuGenTec (PDF)
Categories:- Amides
- Amide solvents
Wikimedia Foundation. 2010.