- Formamide
-
Formamide 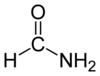
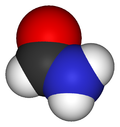 MethanamideOther namesCarbamaldehyde
MethanamideOther namesCarbamaldehyde
FormamideIdentifiers CAS number 75-12-7 
PubChem 713 ChemSpider 693 
KEGG C00488 
ChEBI CHEBI:48431 
ChEMBL CHEMBL266160 
Jmol-3D images Image 1 - O=CN
Properties Molecular formula CH3NO Molar mass 45.04 g/mol Density 1.133 g/cm3 Melting point 2-3 °C, 275-276 K, 36-37 °F
Boiling point 210 °C, 483 K, 410 °F
Solubility in water Miscible Vapor pressure 0.08 mmHg at 20 °C Acidity (pKa) 23.5 (in DMSO)[1] Hazards NFPA 704 Flash point 154 °C (closed cup) Related compounds Related compounds Dimethylformamide  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Formamide, also known as methanamide, is an amide derived from formic acid. It is a clear liquid which is miscible with water and has an ammonia-like odor. It is used primarily for manufacturing sulfa drugs and synthesizing vitamins and as a softener for paper and fiber. In its pure form, it dissolves many ionic compounds that are insoluble in water, so it is also used as a solvent.
Formamide will begin to partially decompose into carbon monoxide and ammonia at 180°C. When heated strongly, formamide decomposes to hydrogen cyanide (HCN) and water vapor.
Contents
Production
In the past, formic acid was reacted with ammonia to produce ammonium formate, which was turned into formamide by heating:
Today's industrial method is based on aminolysis of methyl formate:
with the possibility of the resulting methanol being recycled into more methyl formate, by reaction with formic acid.
While ultimately, the two inputs to this system are identical (formic acid and ammonia), benefit is obtained by virtue of the fact that they can be conducted in separate locations.
Applications
Formamide is also a constituent of cryoprotectant vitrification mixtures used for cryopreservation of tissues and organs.
Formamide is also used as an RNA stabiliser in gel electrophoresis by deionizing RNA. In capillary electrophoresis, it is used for stabilizing (single) strands of denatured DNA.
Another use is to add it in sol-gel solutions in order to avoid cracking during sintering.
Formamide, in its pure state, has been used as an alternative solvent for the electrostatic self-assembly of polymer nanofilms.[2]
Formamide is used to prepare primary amines directly from ketones via their N-formyl derivatives, using the Leuckart reaction.
Self-replication
Main article: Self-replicationFormamide is self-complementary and so can cause dissolved carbon monoxide and ammonia to be reacted to form copies of itself. If heated slightly and in the presence of these chemicals, the formamide will slowly grow in quantity.
Hypothetical Biochemistry
Main article: Hypothetical types of biochemistryFormamide has also been put forward as an alternative solvent to water, perhaps with the ability to support life with alternative biochemistries to that currently found on Earth.[3]
RNA base creation
Formamide has been shown to create guanine at 130 degrees C in the presence of ultra violet light. [4]
Safety
Formamide is highly corrosive on contact with skin or eyes and may be deadly if ingested. Inhalation of large amounts of formamide vapor may require medical attention.[5] It is also a teratogen[6]. Formamide should never be handled without proper safety attire including gloves and goggles. There is a small risk of decompostion into hydrogen cyanide and water.
References
- ^ F. G. Bordwell, J. E. Bartmess and J. A. Hautala (1978). "Alkyl effects on equilibrium acidities of carbon acids in protic and dipolar aprotic media and the gas phase". J. Org. Chem. 43 (16): 3095–3101. doi:10.1021/jo00410a001.
- ^ Vimal K. Kamineni, Yuri M. Lvov, and Tabbetha A. Dobbins (2007). "Layer-by-Layer Nanoassembly of Polyelectrolytes Using Formamide as the Working Medium". Langmuir 23 (14): 7423–7427. doi:10.1021/la700465n. PMID 17536845.
- ^ http://www.astrobio.net/news/article2171.html
- ^ http://www.sciencedaily.com/releases/2010/06/100614101957.htm
- ^ http://hazard.com/msds/mf/baker/baker/files/f5770.htm
- ^ http://www.bath.ac.uk/internal/bio-sci/bbsafe/formamide.htm
Categories:- Amides
- Amide solvents
Wikimedia Foundation. 2010.

