- Hypothetical types of biochemistry
-
Hypothetical types of biochemistry are forms of biochemistry speculated to be scientifically viable but not proven to exist at this time. While the kinds of living beings we know on earth commonly use carbon for basic structural and metabolic functions, water as a solvent and DNA or RNA to define and control their form, it is possible that undiscovered life-forms could exist that differ radically in their basic structures and biochemistry from that known to science.[citation needed]
The possibility of extraterrestrial life being based on these "alternative" biochemistries is a common subject in science fiction, but is also discussed in a non-fiction scientific context. A recent example of the non-fiction discussion is a 2007 report on life's limiting conditions prepared by a committee of scientists under the United States National Research Council.[1] The committee, chaired by John A. Baross, considers "hypothetical alternative chemistries of life",[2] including a range of solvents other than water.[3] The committee begins its discussion by raising the concern that a space agency might conduct a well-resourced search for life on other worlds "and then fail to recognize it if it is encountered".[4]
Alternative-chirality biomolecules
Perhaps the least unusual alternative biochemistry would be one with differing chirality of its biomolecules. In known Earth-based life, amino acids are almost universally of the L form and sugars are of the D form. Molecules of opposite chirality have identical chemical properties to their mirrored forms, so life that used D amino acids or L sugars may be possible; molecules of such a chirality, however, would be incompatible with organisms using the opposing chirality molecules. It is questionable, however, whether such a biochemistry would be truly alien; while it is certainly an alternative stereochemistry, molecules that are overwhelmingly found in one enantiomer throughout the vast majority of organisms can nonetheless often be found in another enantiomer in different (often basal) organisms such as in comparisons between members of Archea and other domains, making it an open topic whether an alternative stereochemistry is truly novel.
Non-carbon-based biochemistries
Scientists have speculated about the pros and cons of using atoms other than carbon to form the molecular structures necessary for life, but no one has proposed a theory employing such atoms to form all the molecular machinery necessary for life. Still, since humans are carbon-based beings and have never encountered life outside the earth’s environment, excluding the possibility of all other elements may be considered carbon chauvinism.
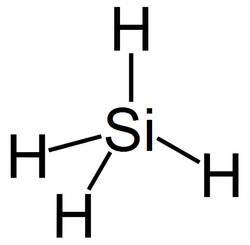
Silicon biochemistry
See also: organosiliconThe most commonly proposed basis for an alternative biochemical system is the silicon atom, since silicon has many chemical properties similar to carbon and is in the same periodic table group, the carbon group. Like carbon, silicon can create molecules that are sufficiently large to carry biological information.[5]
However, silicon has several drawbacks as a carbon alternative. Silicon, unlike carbon, lacks the ability to form chemical bonds with diverse types of atoms, which permits the chemical versatility necessary for metabolism. Elements creating organic functional groups with carbon include hydrogen, oxygen, nitrogen, phosphorus, sulfur, and metals such as iron, magnesium, and zinc. Silicon, on the other hand, interacts with very few other types of atoms.[5] Moreover, where it does interact with other atoms, silicon creates molecules that have been described as "monotonous compared with the combinatorial universe of organic macromolecules".[5] This is because silicon atoms are much bigger, having a larger mass and atomic radius, and so have difficulty forming double or triple covalent bonds, which are important for a biochemical system.
Silanes, which are chemical compounds of hydrogen and silicon that are analogous to the alkane hydrocarbons, are highly reactive with water, and long-chain silanes spontaneously decompose. Molecules incorporating polymers of alternating silicon and oxygen atoms instead of direct bonds between silicon, known collectively as silicones, are much more stable. It has been suggested that silicone-based chemicals would be more stable than equivalent hydrocarbons in a sulfuric-acid-rich environment, as is found in some extraterrestrial locations.[6] Complex long-chain silicone molecules are still less stable than their carbon counterparts, though.
Another obstacle is that silicon dioxide (a common ingredient of many sands), the analog of carbon dioxide, is a non-soluble solid at the temperature range where water is liquid, making it difficult for silicon to be introduced into water-based biochemical systems even if the necessary range of biochemical molecules could be constructed out of it. Another problem with silicon dioxide is that it would be the product of aerobic respiration. If a silicon-based life form were to breathe using oxygen, as life on Earth does, it would possibly produce silicon dioxide as a by-product of this, assuming that the only difference between the two types of life is silicon in place of carbon. This implies that the exhaled product, silicon dioxide, would be a solid, thus filling the respiratory organs of the organism with sand. This however would be solved if the organism lives in temperatures of several hundred to thousand degrees, where the silicon dioxide becomes a liquid. Oxygen-breathing silicon life, if it exists, is therefore most likely to exist in environments with very high temperatures or pressure.
Finally, of the varieties of molecules identified in the interstellar medium as of 1998[update], 84 are based on carbon while only 8 are based on silicon.[7] Moreover, of those 8 compounds, four also include carbon within them. The cosmic abundance of carbon to silicon is roughly 10 to 1. This may suggest a greater variety of complex carbon compounds throughout the cosmos, providing less of a foundation upon which to build silicon-based biologies, at least under the conditions prevalent on the surface of planets.
Also, even though Earth and other terrestrial planets are exceptionally silicon-rich and carbon-poor (the relative abundance of silicon to carbon in the Earth's crust is roughly 925:1[8]), terrestrial life is carbon-based. The fact that carbon, though rare, has proven to be much more successful as a life base than the much more abundant silicon, may be evidence that silicon is poorly suited for biochemistry on Earth-like planets. For example: silicon is less versatile than carbon in forming compounds; the compounds formed by silicon are unstable and it blocks the flow of heat.[9] Even so, biogenic silica is used by some Earth life, such as the silicate skeletal structure of diatoms. This suggests that extraterrestrial life forms may have silicon-based structure molecules and carbon-based proteins for metabolic purposes, therefore enabling the ability to feed on a common resource on a terrestrial planet like Earth for building up the silicon-based part of their body.
Silicon compounds may possibly be biologically useful under temperatures or pressures different from the surface of a terrestrial planet, either in conjunction with or in a role less directly analogous to carbon.
A. G. Cairns-Smith has proposed that the first living organisms to exist on Earth were clay minerals—which were probably based on silicon.[10]
In cinematic and literary science fiction, a moment when man-made machines cross from nonliving to living, it is often posited, this new form would be the first example of non-carbon-based life. Since the advent of the microprocessor in the late 1960s, these machines are often classed as computers (or computer-guided robots) and filed under "silicon-based life", even though the silicon backing matrix of these processors is not nearly as fundamental to their operation as carbon is for "wet life".
Nitrogen and phosphorus biochemistry
Nitrogen and phosphorus also offer possibilities as the basis for biochemical molecules. Like carbon, phosphorus can form long chain molecules on its own, which would potentially allow it to form complex macromolecules were it not so reactive. However, in combination with nitrogen, it can form much more stable covalent bonds and create a wide range of molecules, including rings (a class of compounds called phosphazenes).
Earth's atmosphere is approximately 78% nitrogen, but this would probably not be of much use to a phosphorus-nitrogen (P-N) life-form since molecular nitrogen (N2) is nearly inert and energetically expensive to "fix" due to its triple bond. On the other hand, one could say that some Earth plants such as legumes can fix nitrogen using symbiotic bacteria contained in their root nodules, but those bacteria have to exist before the nitrogen fixation process they perform can actually take place. On Earth, the intense temperatures created by lightning split atmospheric nitrogen in order to make it available for the first nitrogen containing organisms to use. A nitrogen dioxide (NO2) or ammonia (NH3) atmosphere would be more useful. Nitrogen also forms several oxides, such as nitric oxide, nitrous oxide, and dinitrogen tetroxide, and all would be present in a nitrogen-dioxide-rich atmosphere.
- In a nitrogen dioxide atmosphere, P-N plant analogues could absorb nitrogen dioxide from the air and phosphorus from the ground. The nitrogen dioxide would be reduced, with analogues to sugar being produced in the process, and waste oxygen would be released into the atmosphere. Animals based on phosphorus and nitrogen would consume the plants, use atmospheric oxygen to metabolize the sugar analogues, exhaling nitrogen dioxide and depositing phosphorus, or phosphorus-rich material, as solid waste.
- In an ammonia atmosphere, P-N plants would absorb ammonia from the air and phosphorus from the ground, then oxidize the ammonia to produce P-N sugars and release hydrogen waste. P-N animals are now the reducers, breathing in hydrogen and converting the P-N sugars to ammonia and phosphorus. This is the opposite pattern of oxidation and reduction from a nitrogen dioxide world, and from the known biochemistry of Earth. It would be analogous to Earth's atmospheric carbon supply being in the form of methane instead of carbon dioxide.
Debate continues, as several aspects of a phosphorus-nitrogen cycle biology would be energy deficient. Also, nitrogen and phosphorus are unlikely to occur in the ratios and quantity required in the universe. Carbon, being preferentially formed during nuclear fusion, is more abundant and is more likely to end up in a preferred location.
An ammoniated atmosphere would be possible and stable at first view (in a reductive environment), and this type of environment would be preferencially present on massive planets which are more likely to retain hydrogen slowing down its escape to space, and have thick atmosphere that better protect ammonia from radiations; like super-Earth with mass in range between the Earth and the little giant planets like Uranus and Neptune. But it is doubtful that an atmosphere rich in nitrogen dioxide could even exist. Since the nitrogen oxides are all endoenergetic compared with molecular nitrogen and oxygen; and they are oxidizing, they would decompose by stellar radiation and by catalysis on the surface of rocks when they are produced. Unlike nitrogen dioxide, the chemically similar gas nitrogen trifluoride is not endoenergetic and is more stable, but the relative rarity of fluorine means that NF3 is unlikely to be present in large enough concentrations in any atmosphere.
Other exotic element-based biochemistries
- Boron's chemistry is possibly even more variable than that of carbon, since it has the ability to form polyhedral clusters and three-center two-electron bonds. Boranes are dangerously explosive in Earth's atmosphere, but would be more stable in a reducing environment. However, boron's low cosmic abundance makes it less likely as a base for life than carbon.
- Various metals, together with oxygen, can form very complex and thermally stable structures rivaling those of organic compounds; the heteropoly acids are one such family. Some metal oxides are also similar to carbon in their ability to form both nanotube structures and diamond-like crystals (such as cubic zirconia). Titanium, aluminum, magnesium and iron are all more abundant in the Earth's crust than carbon. Metal oxide-based life could therefore be a possibility under certain conditions, including those (such as high temperatures) at which carbon-based life would be unlikely.
- Sulfur is also able to form long-chain molecules, but suffers from the same high reactivity problems as phosphorus and silanes. The biological use of sulfur as an alternative to carbon is purely theoretical, especially since sulfur usually forms only linear chains rather than branched ones. (The biological use of sulfur as an electron acceptor is widespread and can be traced back 3.5 billion years on Earth, thus predating the use of molecular oxygen.[11] Sulfur-reducing bacteria can utilize elemental sulfur instead of oxygen, reducing sulfur to hydrogen sulfide.)
Carbon-based alternatives to hydrocarbons
In hydrogen-depleted or strongly oxidizing environments, a carbon-based biology in which little to no hydrogen is used might be a possibility. Such "hydrogenless life" would use oxocarbons (such as mellitic anhydride or similar) as its building blocks, instead of hydrocarbons.[citation needed]
Chlorine as an alternative to oxygen
A number of alternatives to molecular oxygen as a terminal electron acceptor are known from anaerobic life forms on Earth. However, it has been proposed that chlorine might serve as a more general biological alternative to oxygen, either in carbon-based biologies or hypothetical non-carbon-based ones. But chlorine is much less abundant than oxygen in the universe, and so planets with a sufficiently chlorine-rich atmosphere are likely to be rare, if they exist at all. Chlorine will instead likely be bound up as salts and other inert compounds.
Arsenic as an alternative to phosphorus
Main article: Arsenic biochemistryArsenic, which is chemically similar to phosphorus, while poisonous for most life forms on Earth, is incorporated into the biochemistry of some organisms.[12] Some marine algae incorporate arsenic into complex organic molecules such as arsenosugars and arsenobetaines. Fungi and bacteria can produce volatile methylated arsenic compounds. Arsenate reduction and arsenite oxidation have been observed in microbes (Chrysiogenes arsenatis).[13] Additionally, some prokaryotes can use arsenate as a terminal electron acceptor during anaerobic growth and some can utilize arsenite as an electron donor to generate energy.
It has been speculated that the earliest life forms on Earth may have used arsenic in place of phosphorus in the backbone of their DNA.[14] A common objection to this scenario is that arsenate esters are so much less stable to hydrolysis than corresponding phosphate esters that arsenic would not be suitable for this function.[15] The authors of a 2010 geomicrobiology study supported in part by NASA have postulated that a bacterium, named GFAJ-1, collected in the sediments of Mono Lake in eastern California, can employ such 'arsenic DNA' when cultured without phosphorus.[16][17] They proposed that the bacterium may employ high levels of poly-β-hydroxybutyrate or other means to reduce the effective concentration of water and stabilize its arsenate esters.[17]
This claim was heavily criticized almost immediately after publication for the perceived lack of appropriate controls[18][19] Science writer Carl Zimmer contacted several scientists for an assessment: "I reached out to a dozen experts ... Almost unanimously, they think the NASA scientists have failed to make their case".[20]
Selenium or tellurium as an alternative to sulfur
Some organisms are already known to feature selenoproteins, in which sulfur is replaced by selenium. Some fungi also can produce telluro-methionine and telluro-cysteine. No organism has yet been shown to completely replace sulfur with the heavier chalcogens, however.
Non-water solvents
In addition to carbon compounds, all currently known terrestrial life also requires water as a solvent. This has led to discussion about whether water is the only liquid capable of filling that role. The idea that an extraterrestrial life-form might be based on a solvent other than water has been taken seriously in recent scientific literature by the biochemist Steven Benner,[21] and by the astrobiological committee chaired by John A. Baross.[3] Solvents discussed by the Baross committee include ammonia,[22] sulfuric acid,[23] formamide,[24] hydrocarbons,[24] and (at temperatures much lower than Earth's) liquid nitrogen, or hydrogen in the form of a supercritical fluid.[25]
Carl Sagan once described himself as both a carbon chauvinist and a water chauvinist;[26] however on another occasion he said he was a carbon chauvinist but "not that much of a water chauvinist".[27] He considered hydrocarbons,[27] hydrofluoric acid,[28] and ammonia [27][28] as possible alternatives to water.
Some of the properties of water that are important for life processes include a large temperature range over which it is liquid, a high heat capacity useful for temperature regulation, a large heat of vaporization, and the ability to dissolve a wide variety of compounds. Water is also amphoteric, meaning it can donate and accept an H+ ion allowing it to act as an acid or a base. This property is crucial in many organic and biochemical reactions, where water serves as a solvent, a reactant, or a product. There are other chemicals with similar properties that have sometimes been proposed as alternatives. Additionally, water is the only compound listed here that is less dense as a solid (ice) than as a liquid. This is why bodies of water freeze over but do not freeze solid (from the bottom up). If ice were denser than liquid water (as is true for nearly all other compounds) then large bodies of liquid would slowly freeze solid, which would not be conducive to the formation of life.
There are some properties that make certain compounds and elements much more favorable than others as solvents in a successful biosphere. The solvent must be able to exist in liquid equilibrium over a range of temperatures the planetary object would normally encounter. Since boiling points can vary based on the pressure, the question tends not to be does the prospective solvent remain liquid, but at what pressure. For example, hydrogen cyanide has a narrow liquid phase temperature range at 1 atmosphere, but in an atmosphere with the pressure of Venus, with 92 bars (9.2 MPa) of pressure, it can indeed exist in liquid form over a wide temperature range.
Ammonia
Ammonia is perhaps the most commonly proposed alternative. Numerous chemical reactions are possible in an ammonia solution, and liquid ammonia has some chemical similarities with water. Ammonia can dissolve most organic molecules at least as well as water does, and in addition it is capable of dissolving many elemental metals. Given this set of chemical properties it has been theorized that ammonia-based life forms might be possible.
However, ammonia has some problems as a basis for life. The hydrogen bonds between ammonia molecules are weaker than those in water, causing ammonia's heat of vaporization to be half that of water, its surface tension to be three times smaller, and reducing its ability to concentrate non-polar molecules through a hydrophobic effect. For these reasons, some members of the scientific community[who?] question how well ammonia could hold prebiotic molecules together to allow the emergence of a self-reproducing system. Ammonia is also flammable and can be oxidized and could not exist sustainably in a biosphere that oxidizes it. It would, however, be stable in a reducing environment.[citation needed]
A biosphere based on ammonia would likely exist at temperatures or air pressures that are extremely unusual for terrestrial life on Earth. Terrestrial life on Earth usually exists within the melting point and boiling point of water at normal pressure, between 0 °C (273 K) and 100 °C (373 K); at normal pressure ammonia's melting and boiling points are between −78 °C (195 K) and −33 °C (240 K). Such extremely cold temperatures create problems, as they slow biochemical reactions tremendously and may cause biochemical precipitation out of solution due to high melting points. Ammonia could be a liquid at normal temperatures, but at much higher pressures; for example, at 60 atm, ammonia melts at −77 °C (196 K) and boils at 98 °C (371 K).[citation needed]
Ammonia and ammonia-water mixtures remain liquid at temperatures far below the freezing point of pure water, so such biochemistries might be well suited to planets and moons orbiting outside the water-based habitability zone. Such conditions could exist, for example, under the surface of Saturn's largest moon Titan.[29]
Methane
A mixture of hydrocarbons, such as the methane/ethane lakes detected on Titan by the Cassini spacecraft, could act as a solvent over a wide range of temperatures but would lack polarity. There is debate about the effectiveness of methane as a medium for life compared to water or ammonia.[30] While water is a far better solvent than methane, enabling easier transport of substances in a cell,[31] methane's lesser chemical reactivity allows for the easier formation of large structures akin to proteins.[32] Isaac Asimov, the biochemist and science fiction writer, suggested that poly-lipids could form a substitute for proteins in a non-polar solvent such as methane or liquid hydrogen.[33]
Possible evidence for this form of life on Titan was identified in 2010 by Darrell Strobel of Johns Hopkins University; an over-abundance of molecular hydrogen in Titan's upper atmospheric layers, which leads to a downward flow at a rate of roughly 1025 molecules per second. Near the surface the hydrogen apparently disappears, which may imply its consumption by methanogenic life forms.[31][34][35] Another paper released the same month showed little evidence of acetylene on Titan's surface, where scientists had expected the compound to accumulate; according Strobel, this is consistent with the hypothesis that acetylene is being consumed by methanogens.[31] Chris McKay, while agreeing that presence of life is a possible explanation for the findings about hydrogen and acetylene, has cautioned that other explanations are currently more likely: namely the possibility that the results are due to human error, or to the presence of some as-yet unknown catalyst in the soil.[36] He noted that such a catalyst, effective at 95 Kelvin, would in itself be a startling discovery.[36]
Hydrogen fluoride
Hydrogen fluoride, like water, is a polar molecule, and due to its polarity it can dissolve many ionic compounds. Its melting point is −84 °C and its boiling point is 19.54 °C (at atmospheric pressure); the difference between the two is little more than 100 °C. HF also makes hydrogen bonds with its neighbor molecules as do water and ammonia. These would make HF a candidate to host life on other planets.
Not much research has been done on liquid HF in regards to its ability to dissolve and react with non-polar molecules. The biota in an HF ocean could use the fluorine as an electron acceptor to photosynthesize energy.
HF is dangerous to the systems of molecules that earth-life is made of, but the paraffins are stable with it.[28]
But presence of great amounts of free HF on a planetary scale, like water on Earth, is only possible outside the temperature range from liquid water and water vapour. Any free water would be react with the polar HF and would form a solution of hydrofluoric acid. The hypothetical planet would also surely contain silicates that would react with HF to form inert compounds as silicon fluorides, as soon as HF would be present; thus preventing its concentration in great quantities in a hypothetical planetary environment.
The cosmic abundance of fluorine is low, and it forms chemically inert compounds rapidly in interstellar nebulae, because it is the most reactive element.
Other solvents or cosolvents
Other solvents sometimes proposed include formamide, methanol, hydrogen sulfide and hydrogen chloride. Hydrogen chloride suffers from the low cosmic abundance of chlorine, while hydrogen sulfide suffers from its high reactivity. Moreover, the first two could not be expected to be found in vast quantities on a planetary scale, and would only be part of the internal physiology of organisms.
A proposal has been made that life on Mars may exist and be using a mixture of water and hydrogen peroxide as its solvent. A 61.2% (by weight) mix of water and hydrogen peroxide has a freezing point of −56.5 °C, and also tends to super-cool rather than crystallize. It is also hygroscopic, an advantage in a water-scarce environment.[37][38]
Molten salts
A molten salt, or a eutectic mixture of such salts, could remain liquid at extremely high temperatures,[specify] and could theoretically act as a solvent. Life in such circumstances may be restricted to refractory materials like metal oxides. The participation of metal oxide compounds in a biochemical system is unlikely, as their molecular structures are simpler compared to organic compounds and they are largely inert.
Other types of speculations
Non-green photosynthesizers
Physicists have noted that, while photosynthesis on Earth generally involves green plants, a variety of other colored plants could also support photosynthesis, essential for most life on Earth, and that other colors might be preferred in places that receive a different mix of stellar radiation than that received on Earth.[39][40] These studies indicate that while blue photosynthetic plants would be unlikely (because absorbed blue light provides some of the highest photosynthetic yields in the light spectrum[citation needed]), yellow or red plants are plausible. These conclusions are based in part on the luminosity spectra of different types of stars, the transmission characteristics of hypothetical planetary atmospheres, and the absorption spectra of various photosynthetic pigments from organisms on Earth.
The first plants on Earth may have been a slightly different colour, because the Sun was, in the first geological eons, a little less bright, and its light was filtered by passing through an atmosphere with a different composition.
Black is the optimum color for converting all available light to energy as efficiently as possible. It is as of yet unclear exactly why plants on Earth are green and not black.
Alternative atmospheres
The gases present in the atmosphere on Earth have varied greatly over its history. Traditional plant photosynthesis has terraformed the atmosphere by sequestering carbon from carbon dioxide, increasing the proportion of molecular oxygen, and by participating in the nitrogen cycle. Modern oxygen breathing animals would have been biochemically impossible until earlier photosynthetic life transformed Earth's atmosphere. The first dramatic rise in atmospheric oxygen on Earth, to about a tenth of its present-day value, occurred approximately 2.5 billion years ago, and that level did not change significantly until the Cambrian era approximately 600 million years ago.[41]
Changes in the gas mixture in the atmosphere, even in an atmosphere made up predominantly of the same molecules of Earth's atmosphere, impacts the biochemistry and morphology of life. For example, periods of high oxygen concentrations determined from ice core samples have been associated with fauna of a larger scale in the fossil record, while periods associated with of low oxygen concentrations have been associated with fauna of a smaller scale in the fossil record.[42]
Also, while it is customary to think of plants on one side of the oxygen and nitrogen cycles as being sessile, and of animals on the other side as being motile, this is not a biological imperative. There are animals which are sessile for all or most of their lives (such as corals), and there are plants (such as tumbleweeds, and venus fly traps) that exhibit more mobility than is customarily associated with plants. On a slowly rotating planet, for example, it might be adaptive for photosynthesis to be performed by "plants" that can move to remain in the light, like Earth's sunflowers; while non-photosynthetic "animals", much like Earth's fungi, might have a lesser need to move from place to place on their own. This would be a mirror image of Earth's ecology.
Variable environments
Many Earth plants and animals undergo major biochemical changes during their life cycles as a response to changing environmental conditions, for example, by having a spore or hibernation state that can be sustained for years or even millennia between more active life stages. Thus, it would be biochemically possible to sustain life in environments that are only periodically consistent with life as we know it.
For example, frogs in cold climates can survive for extended periods of time with most of their body water in a frozen state,[43] while desert frogs in Australia can become inactive and dehydrate in dry periods, losing up to 75% of their fluids, yet return to life by rapidly rehydrating in wet periods.[44] Either type of frog would appear biochemically inactive (i.e. not living) during dormant periods to anyone lacking a sensitive means of detecting low levels of metabolism.
Nonplanetary life
Dust and plasma-based
In 2007 V. N. Tsytovich and colleagues proposed that life-like behaviors could be exhibited by dust particles suspended in a plasma, under conditions that might exist in space.[45][46] Computer models showed that when the dust became charged the particles could self-organize into microscopic helical structures capable of replicating themselves, interacting with other neighboring structures, and evolving into more stable forms. Similar forms of life were described in Fred Hoyle's classic novel The Black Cloud.
Usage in fiction
In the realm of science fiction, there have occasionally been forms of life proposed that, while often highly speculative and unsupported by rigorous theoretical examination, are nevertheless interesting and in some cases even plausible.
Novels, short stories and comics
In Arthur C. Clarke's short story "Technical Error" there is an example of differing chirality. This is not a case of alien life, rather it is an accident. A technical error in a new power plant changes the chirality of a worker. He cannot read because he sees all type as reversed, and he believes his left side is his right. The amusement ends when it is discovered that he is dying from malnutrition because his new biology cannot utilize normal food.
The concept of reversed chirality also figured prominently in the plot of James Blish's Star Trek novel Spock Must Die!, where a transporter experiment gone awry ends up creating a duplicate Spock who turns out to be a perfect mirror-image of the original all the way down to the atomic level.
An example of silicon based life forms takes place in the Alan Dean Foster novel Sentenced to Prism in which the protagonist Evan Orgell is trapped on a planet whose entire ecosystem is mostly silicon-based.
Perhaps the most extreme example in science fiction is James White's Sector General: a series of novels and short stories about multienvironment hospital for the strangest life-forms imaginable, some of them breathing methane, chlorine, water and sometimes also oxygen. Some of the species metabolise directly hard radiation and their environment doesn't differ much from the atmosphere of a star, while others live in near absolute zero temperatures. All life forms are classified according to their metabolism, internal and external features, and more extreme abilities (telepathy, empathy, hive mind, etc.) with four letter codes. Humans from Earth share the DBDG specification with small furry beings called Nidians.
One of the major sentient species in Terry Pratchett's Discworld universe are the "earth"-based (ranging from Detritus to Diamond) Trolls.
Pratchett has also written the science fiction novel The Dark Side of the Sun which features a range of extraordinary life-forms, including a telepathic body of water, creatures called "Sundogs", which are capable of interstellar travel from birth, and a sentient planet: effectively a giant silicon-based computer.
Fred Hoyle's classic novel The Black Cloud features a life form consisting of a vast cloud of interstellar dust, the individual particles of which interact via electromagnetic signalling analogous to how the individual cells of multicellular terrestrial life interact. Outside of science-fiction, life in interstellar dust has been proposed as part of the panspermia hypothesis. The low temperatures and densities of interstellar clouds would seem to imply that life processes would operate much more slowly there than on Earth. Inorganic dust-based life has been speculated upon based on recent computer simulations.[46]
Similarly, Arthur C. Clarke's "Crusade" revolves around a planetwide life-form based on silicon and superfluid helium located in deep intergalactic space, processing its thoughts slowly by human standards, that sends probes to look for similar life in nearby galaxies. It concludes that it needs to make planets more habitable for similar life-forms, and sends out other probes to foment supernovae to do so. Clarke implies that this is what accounts for most supernovae having occurred in the same region of space and warns that the effort will eventually reach Earth.
Robert L. Forward's Camelot 30K describes an ecosystem on the surface of Kuiper belt objects that is based on a fluorocarbon chemistry with OF2 as the principal solvent instead of H2O. The organisms in this ecology keep warm by secreting a pellet of uranium-235 inside themselves and then moderating its nuclear fission using a boron-rich carapace around it. Kuiper belt objects are known to be rich in organic compounds such as tholins, so some form of life existing on their surfaces is not entirely implausible–though perhaps not going so far as to develop natural internal nuclear reactors, as have Forward's. Fluorine is also of low cosmic abundance, so its use in this manner is unlikely.
In Forward's Rocheworld series, an Earth-like biochemistry is proposed that uses a mixture of water and ammonia as its solvent. In Dragon's Egg and Starquake, Forward proposes life on the surface of a neutron star utilizing "nuclear chemistry" in the degenerate matter crust. Since such life utilised strong nuclear forces instead of electromagnetic interactions, it was posited that life might function millions of times faster than typical on Earth.
Gregory Benford and David Brin's Heart of the Comet features a comet with a conventional carbon-and-water-based ecosystem that becomes active near the perihelion when the Sun warms it. Brin's own novel Sundiver is an example of science fiction proposing a form of life existing within the plasma atmosphere of a star using complex self-sustaining magnetic fields. Similar sorts of plasmoid life have sometimes been proposed to exist in other places, such as planetary ionospheres or the interstellar medium, but usually only by fringe theorists (see ball lightning for some additional discussion).[citation needed] Gregory Benford had a form of plasma-based life exist in the accretion disk of a primordial black hole in his novel Eater.
The suggestion that life could even occur within the plasma of a star has been picked up by other science fiction writers, as in David Brin's Uplift Saga or Frederik Pohl's novel The World at the End of Time. The idea is that places where reactions occur–even an incredible environment as a star–presents a possible medium for some chain of events that could produce a system able to replicate.
The Outsiders in Larry Niven's Known Space universe are cryogenic creatures based on liquid helium. They derive thermoelectric energy from a temperature gradient by basking half their body in sunlight, keeping the other half in shadow and exposed to interstellar vacuum.
Stephen Baxter has imagined perhaps some of the most unusual exotic life-forms in his Xeelee series of novels and stories, including supersymmetric photino-based life that congregate in the gravity wells of stars, entities composed of quantum wave functions, and the Qax, who thrive in any form of convection cells, from swamp gas to the atmospheres of gas giants. In his book Manifold: Space, he also proposes natural robots, life forms made of iron, called the Gaijin, evolving from creatures in oceans of iron carbonyl.
In his novel Diaspora, Greg Egan posits entire virtual universes implemented on Turing Machines encoded by Wang Tiles in gargantuan polysaccharide 'carpets.' The sentient ocean that covers much of the surface of Solaris in Stanislaw Lem's eponymous novel also seems, from much of the fictional research quoted and discussed in the book, to be based on some element other than carbon. In the same novel Egan describes lifeforms in the 6-D 'macrosphere' which use a collapsed atom chemistry with energetic processes of the same order as nuclear reactions, due to the peculiarities of higher dimensional physics.
In her novel Brain Plague, Joan Slonczewski describes a species of intelligent microrganisms with arsenic based chemistries that live symbiotically with human hosts.
Sergeant Schlock is one of the lead characters in the webcomic Schlock Mercenary. His species, Carbosilicate Amorphs, evolved from self-repairing distributed data storage devices, and as such, redundantly distribute their 'brain' throughout their body. They are highly resistant to hard vacuum, explosive decompression, projectile weapons, chemical-based explosives, and dismemberment. Their only specialty organ is their eyes, which they harvest as fruit from the Ghanj-Rho eye-tree on their home planet. While the Amorphs have the ability to move fast, quietly,[47] and sprout appendages at will, they excel at 'closer-than-melee-range combat, primarily "meme-toxins" against other Amorphs.
A more farcical example comes from The Hitchhiker's Guide to the Galaxy, where the Hooloovoo are a hyperintelligent shade of the colour blue.
Alien warriors recruited by the god Klael in David Eddings' "Tamuli" trilogy are noted by their human opponents to breathe marsh-gas (methane). Within Eddings' universe, this limits their capacity for exertion in an oxygen atmosphere, and also determines the tactics used to fight them and eventually to destroy them in their encampments.
The eponymous organism in Michael Crichton's The Andromeda Strain is described as reproducing via the direct conversion of energy into matter.
Star Trek
A well-known example of a non–carbon-based life-form in science fiction is the Horta in the original Star Trek episode "Devil in the Dark". A highly intelligent silicon-based creature made almost entirely of pure rock, it tunnels through rock as easily as humans move through air. The entire species dies out every 50,000 years except for one who tends for all the eggs, which take the form of silicon nodules scattered throughout the caverns and tunnels of its home planet, Janus VI. The inadvertent destruction of many of these eggs by a human mining colony led the mother Horta to respond by killing the colonists and sabotaging their equipment; it was only through a Vulcan mind meld that the race's benevolence and intelligence were discovered and peaceful relations established.
Star Trek would later offer other corporeal life-forms with an alternative biochemistry. The Tholians of "The Tholian Web" are depicted and described, in that episode and later in the Star Trek: Enterprise episode "In a Mirror, Darkly" as being primarily of mineral-based composition and thriving only in superheated conditions. Another episode from TOS's third season, "The Savage Curtain", depicted another rock creature called an Excalbian, which is believed in fanon to also have been silicon-based.[48]' [49]
In Star Trek: The Next Generation, the Crystalline Entity appeared in two episodes, "Datalore" and "Silicon Avatar". This was an enormous spacefaring crystal lattice that had taken thousands of lives in its quest for energy. It may have been unaware of this, however, but it was destroyed before communications could be established at a level sufficient to ascertain it.
In another episode, "Home Soil", intelligent crystals that formed a "microbrain" were discovered during a terraforming mission, and they described the humans they encountered as "ugly bags of mostly water."
"The Disease", an episode of Star Trek: Voyager featured some artificially-engineered silicon-based parasites, and an Enterprise episode, "Observer Effect", also presented a lethal silicon-based virus. In another Voyager episode, "Hope and Fear", a xenon-based life-form was mentioned. In the Enterprise episode "The Communicator", an alien species is encountered whose blood chemistry, while not explicitly stated, is sufficiently different from terrestrial organisms that it is not red and iron is toxic to it. Various Star Trek series also had episodes featuring photonic lifeforms.
Star Wars
In the Star Wars movie The Empire Strikes Back, two life-forms were encountered by the characters that were non-carbon based entities. Although details of their physiology were not mentioned on screen, the space slug, (a giant worm-like creature that lived on asteroids in the vacuum of space),[50][51] and the Mynock, (pesky bat-like vermin that would attach to spaceship hulls and chew through power conduits to feed off the raw energy),[52][53] are said to be silicon-based organisms in Star Wars Expanded Universe sources. Also from The Empire Strikes Back, the bounty hunter Zuckuss is a member of the Gand race, an ammonia-based life-form. However, it is worth noting that the Gand are divided into two subspecies, only one of which breathes at all, the other drawing all their required sustenance from food intake and producing speech by essentially modulated flatulence.
Appearing only in the Star Wars Expanded Universe is the spice spider of Kessel, a creature made of glitterstim spice and silicon that spun crystalline webs harvested by miners as glitterstim spice, an illegal psychoactive narcotic. The spider used the webs to catch bogeys, tiny energy creatures that it consumed for energy.[54][55]
Other film and television
- In the movie Titan A.E., an alien race called the Drej were composed of a coherent plasma.
- In the movie The Monolith Monsters, a silicon meteor reproduces, draining silicates from everything it touches. It needs water to start its cycle and contains molecular structures typical of many kinds of rocks, mixed together. A geologist says that its structure is nearly impossible. The meteor is killed by salt water, which can stop the cycle.
- In Firewalker, a second-season episode of The X-Files, a silicon-based plant that infects humans parasitically through its spores is discovered living deep in a volcano.
- Also from The X-Files, the first-season episode "Ice" deals with an ammonia-based vermiform parasite.
- A key plot point in the comedy Evolution involves nitrogen-based life forms, and using selenium-based shampoo to poison them (with the bonus of a product placement for Head & Shoulders).
- In the Stargate SG-1 fourth season episode "Scorched Earth", a Human society known as the Enkarans are threatened on their new homeworld by an alien ship that is terraforming the planet to be suitable for the sulfur-based Gadmeer species.
- In the Stargate Atlantis fifth season episode "Remnants", a device is found whose purpose was to seed a planet with silicon-based life.
- In Ben 10, both the Omnitrix alien Diamondhead and the alien bounty hunter Tetrax Shard are members of the Petrosapien species, which are a form of silicon-based life. Other silicon-based lifeforms on the show include the Omnitrix aliens Chromostone (who is crystalline), and Echo Echo and Upgrade (who are both biomechanical). Other member's of Upgrade's species have appeared, including the shape-shifting "Ship," a pet of Ben's girlfriend, Julie.
- In Dragon Ball Z, shortly before the destruction of Planet Namek, Frieza tells Goku that he does not need to breathe. This would allow him to survive the destruction of the planet and suggest that he is not a carbon-based life-form.
- Indiana Jones and the Kingdom of the Crystal Skull (2008) introduces thirteen "extra dimensional beings" with crystal skeletons, who founded a city that became the basis of the El Dorado myth. Though their flesh has died and rotted away, their minds still live on within their skeletons, which communicate telepathically.
- An episode ("The Chrysalids") of the series Space: 1999 presents humanoids on a planet protected by a electric waves system, which are living in a chlorine atmosphere.
- The episode "The Stones of Blood", of the 16th season of Doctor Who, the Fourth Doctor encounters the Ogri, a silicon-based life form, and in the same sub-plot, the Megara, who are made entirely out of an unknown substance, possibly energy, and they uphold the word of the law, and execute all who break the law with a beam of energy.
Computer and video games
In the Command & Conquer real-time strategy games, both the gameplay and storyline revolve heavily around the introduction to Earth of an extraterrestrial mutagen called Tiberium via meteor, which displays strikingly lifelike behaviours such as self-replication, evolution, and homeostasis, without undergoing anything like common carbon-based metabolic cycles, and which appears to be colonising the Earth, converting it into an environment unsuited to carbon-based biology. Earth creatures (such as animals, plants and even humans) exposed to Tiberium can either be killed because of the radiation or be transformed into Tiberium-based life-forms, to whom Tiberium radiation is curative rather than toxic. It is later revealed that Tiberium was introduced to earth by the Scrin, an extremely advanced race of Tiberium-based aliens bent on mining the planet after the Tiberium deposits have reached maturity.
In the Halo video game series, a race of Covenant aliens named "Grunts" by humans require a breathing apparatus while fighting the humans in an Earth-like atmosphere. According to the novelizations of the video game, the Grunts' apparatus allows them to breathe the methane they need to survive.
In the Master of Orion series of space strategy games, there exists an extraterrestrial race called Silicoids, whose appearance (and presumably composition) is similar to crystalline mineral structures. The game posits that this grants them immunity to the effects of hostile environments and pollution and they require no sustenance, at the expense of impeding their reproductive rate and their ability to interact with other intelligent species.
In the Metroid Prime series, Phazon is a highly radioactive, self-regenerating mineral with organic properties that is generated by the sentient planet Phaaze.
In Metroid Prime Hunters, Spire is a rock-like, silicon-based alien. He is the last Diamont (presumably a play on the word diamond, which is composed of carbon).
In the Submarine TITANS strategy game, the alien race in the game are called "the Silicons" because they are silicon-based life forms.
In the Star Control series, the Chenjesu, are hyperintelligent, peaceful silicon-based life-forms that were the backbone of the Alliance of Free Stars. Their crystalline biology apparently gives them the ability to send and receive hyperwave transmissions. Also, there are the Slylandro, who are gas beings residing in the upper atmosphere of a gas giant. As well, there are evidences of another silicon-based race, the Taalo who are described by the xenophobic Ur-Quan as the only race to have not awakened their territorial instincts. The Taalo were also immune to mind control
In the game of Xenosaga, artificial life forms known as Realians have been created using silicon-based chemistry. They resemble humans in every aspect, except they are considered to be lower than humans on the social ladder.
In Mass Effect the alien Turians and Quarians, are both based on dextro-amino acids, as opposed to the all other sentient species of the galaxy based on levo-amino acids. There are also the Volus, an ammonia based species that must wear pressure suits to survive in environments suited to the other races.
In Spore, the Grox refer to the player and to other alien empires as "slow thinking carbon-based lifeforms" and "carbon wads", implying that the Grox (which are at least partly machine life) are not carbon-based. Also, the Grox can only exist on barren planets which cannot support other life, and when a planet is terraformed the Grox inhabiting it die immediately. The Grox seem to gather sustenance from the radiation from the galactic core, as the Grox colonies are larger the closer they are to the galactic core.
In Muv Luv, the BETA which calls itself the "higher/superior existence" says they were created by a silicon-based being simply called "The Creator". As such, they don't consider any non-silicon-based creature to be alive, not even themselves. Its reasoning was that only silicon-based beings occur naturally and have the ability to reproduce and disperse. When the human main character, Takeru, argues that humans also have the ability to reproduce and disperse, the higher existence says carbon too easily mingles with other elements and therefore it would be impossible for a carbon-based existence to have evolved on its own. Thus, humans must be other biological machines created by a life form just as the BETA are.
See also
References
- ^ Committee on the Limits of Organic Life in Planetary Systems, Committee on the Origins and Evolution of Life, National Research Council; The Limits of Organic Life in Planetary Systems; The National Academies Press, 2007.
- ^ Committee on the Limits of Organic Life in Planetary Systems, Committee on the Origins and Evolution of Life, National Research Council; The Limits of Organic Life in Planetary Systems; The National Academies Press, 2007; page x
- ^ a b Committee on the Limits of Organic Life in Planetary Systems, Committee on the Origins and Evolution of Life, National Research Council; The Limits of Organic Life in Planetary Systems; The National Academies Press, 2007; pages 69 - 79
- ^ Committee on the Limits of Organic Life in Planetary Systems, Committee on the Origins and Evolution of Life, National Research Council; The Limits of Organic Life in Planetary Systems; The National Academies Press, 2007; page 5
- ^ a b c Pace, NR (2001). "The universal nature of biochemistry.". Proceedings of the National Academy of Sciences of the United States of America 98 (3): 805–8. Bibcode 2001PNAS...98..805P. doi:10.1073/pnas.98.3.805. PMC 33372. PMID 11158550. http://www.pnas.org/content/98/3/805.full.pdf.
- ^ Gillette, Stephen. World-Building. Writer's Digest Books. ISBN 0898797071.
- ^ Lazio, Joseph. "F.10 Why do we assume that other beings must be based on carbon? Why couldn't organisms be based on other substances?". [sci.astro] ET Life (Astronomy Frequently Asked Questions). http://www.faqs.org/faqs/astronomy/faq/part6/section-16.html. Retrieved 2006-07-21.
- ^ "Abundance in Earth's Crust". WebElements.com. http://www.webelements.com/webelements/properties/text/image-flash/abund-crust.html. Retrieved 2007-04-14.
- ^ "Astrobiology". Biology Cabinet. September 26, 2006. http://biocab.org/Astrobiology.html. Retrieved 2011-01-17.
- ^ Cairns-Smith, A. Graham (1985). Seven Clues to the Origin of Life. Cambridge: Cambridge University Press. ISBN 0-521-27522-9.
- ^ Early Archaean Microorganisms Preferred Elemental Sulfur, Not Sulfate Science AAAS, by Philippot, et al., (14 September 2007)
- ^ "Biochemical Periodic Table – Arsenic". Umbbd.msi.umn.edu. 2007-06-08. http://umbbd.msi.umn.edu/periodic/elements/as.html. Retrieved 2010-05-29.
- ^ Niggemyer, A; Spring S, Stackebrandt E, Rosenzweig RF (December 2001). "Isolation and characterization of a novel As(V)-reducing bacterium: implications for arsenic mobilization and the genus Desulfitobacterium". Appl Environ Microbiol 67 (12): 5568–80. doi:10.1128/AEM.67.12.5568-5580.2001. PMC 93345. PMID 11722908. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=93345.
- ^ Reilly, Michael (26 April 2008). "Early life could have relied on 'arsenic DNA'". New Scientist 198 (2653): 10. doi:10.1016/S0262-4079(08)61007-6. http://www.newscientist.com/channel/life/mg19826533.600-early-life-could-have-relied-on-arsenic-dna.html.
- ^ Westheimer, F. H. (1987-03-06). "Why nature chose phosphates". Science 235 (4793): 1173–1178 (see pp. 1175–1176). Bibcode 1987Sci...235.1173W. doi:10.1126/science.2434996. http://academic.evergreen.edu/curricular/m2o2006/seminar/westheimer.pdf. Retrieved 2010-12-03.
- ^ "NASA-Funded Research Discovers Life Built With Toxic Chemical". NASA.gov. 2 December 2010. http://www.nasa.gov/topics/universe/features/astrobiology_toxic_chemical.html. Retrieved 2010-12-02.
- ^ a b Wolfe-Simon, Felisa; Blum, Jodi Switzer; Kulp, Thomas R.; Gordon, Shelley E.; Hoeft, S. E.; Pett-Ridge, Jennifer; Stolz, John F.; Webb, Samuel M. et al. (2 December 2010). "A Bacterium That Can Grow by Using Arsenic Instead of Phosphorus" (PDF). Science 332 (6034): 1163–6. Bibcode 2011Sci...332.1163W. doi:10.1126/science.1197258. PMID 21127214. http://www.ironlisa.com/WolfeSimon_etal_Science2010.pdf. Retrieved 2010-12-09.
- ^ Redfield, Rosemary (4 December 2010). "Arsenic-associated bacteria (NASA's claims)". RR Research blog. http://rrresearch.blogspot.com/2010/12/arsenic-associated-bacteria-nasas.html. Retrieved 4 December 2010.
- ^ Bradley, Alex (5 December 2010). "Arsenate-based DNA: a big idea with big holes". We, Beasties blog. http://scienceblogs.com/webeasties/2010/12/guest_post_arsenate-based_dna.php. Retrieved 9 December 2010.
- ^ Zimmer, Carl (7 December 2010). "Scientists see fatal flaws in the NASA study of arsenic-based life". Slate. http://www.slate.com/id/2276919/. Retrieved 7 December 2010.
- ^ Benner, Steven A. et al (2004). "Is there a common chemical model for life in the universe?". Current Opinion in Chemical Biology 8: 676–680.Text as pdf from www.sciencedirect.com (accessed 13 July 2011)
- ^ Committee on the Limits of Organic Life in Planetary Systems, Committee on the Origins and Evolution of Life, National Research Council; The Limits of Organic Life in Planetary Systems; The National Academies Press, 2007; p 72
- ^ Committee on the Limits of Organic Life in Planetary Systems, Committee on the Origins and Evolution of Life, National Research Council; The Limits of Organic Life in Planetary Systems; The National Academies Press, 2007; p 73
- ^ a b Committee on the Limits of Organic Life in Planetary Systems, Committee on the Origins and Evolution of Life, National Research Council; The Limits of Organic Life in Planetary Systems; The National Academies Press, 2007; p 74
- ^ Committee on the Limits of Organic Life in Planetary Systems, Committee on the Origins and Evolution of Life, National Research Council; The Limits of Organic Life in Planetary Systems; The National Academies Press, 2007; p 75
- ^ Sagan, Carl (2002). Cosmos. Random House. pp. 126–127. ISBN 0375508325.
- ^ a b c Sagan, Carl; Head, Tom (2006). Conversations with Carl Sagan. University Press of Mississipi. p. 10. ISBN 1578067367.
- ^ a b c Sagan, Carl (2002). Cosmos. Random House. p. 128. ISBN 0375508325.
- ^ Fortes, A. D. (1999). "Exobiological Implications of a Possible Ammonia-Water Ocean Inside Titan". http://www.es.ucl.ac.uk/research/planetary/undergraduate/dom/titan/titan.htm. Retrieved 7 June 2010.
- ^ Committee on the Limits of Organic Life in Planetary Systems, Committee on the Origins and Evolution of Life, National Research Council; The Limits of Organic Life in Planetary Systems; The National Academies Press, 2007; page 74.
- ^ a b c "What is Consuming Hydrogen and Acetylene on Titan?". NASA/JPL. 2010. http://www.jpl.nasa.gov/news/news.cfm?release=2010-190. Retrieved 2010-06-06.
- ^ Steven A Benner�, Alonso Ricardo and Matthew A Carrigan (2004). "Is there a common chemical model for life in the universe?". Current Opinion in Chemical Biology 8 (6): 672–689. doi:10.1016/j.cbpa.2004.10.003. PMID 15556414. http://www.ffame.org/sbenner/cochembiol8.672-689.pdf. Retrieved 2010-06-14.
- ^ Chung, Winchell D. jr. (2011-05-30). "Aliens". Atomic Rockets. Projectrho.com. http://www.projectrho.com/rocket/aliens.php. Retrieved 2011-07-04.
- ^ Darrell F. Strobel (2010). In press. "Molecular hydrogen in Titan’s atmosphere: Implications of the measured tropospheric and thermospheric mole fractions". Icarus 208 (2): 878. Bibcode 2010Icar..208..878S. doi:10.1016/j.icarus.2010.03.003.
- ^ McKay, C. P.; Smith, H. D. (2005). "Possibilities for methanogenic life in liquid methane on the surface of Titan". Icarus 178 (1): 274–276. Bibcode 2005Icar..178..274M. doi:10.1016/j.icarus.2005.05.018.
- ^ a b Chris Mckay (2010). "Have We Discovered Evidence For Life On Titan". SpaceDaily. http://www.spacedaily.com/reports/Have_We_Discovered_Evidence_For_Life_On_Titan_999.html. Retrieved 2010-06-10.
- ^ Houtkooper, Joop M.; Dirk Schulze-Makuch (2007). "The H2O2-H2O Hypothesis: Extremophiles Adapted to Conditions on Mars?" (PDF). EPSC Abstracts (European Planetary Science Congress 2007) 2. EPSC2007-A-00439. http://www.cosis.net/abstracts/EPSC2007/00439/EPSC2007-J-00439.pdf.
- ^ Ellison, Doug (2007-08-24). "Europlanet : Life's a bleach". Planetary.org. http://www.planetary.org/blog/article/00001109/.
- ^ "NASA – NASA Predicts Non-Green Plants on Other Planets". Nasa.gov. 2008-02-23. http://www.nasa.gov/centers/goddard/news/topstory/2007/spectrum_plants.html. Retrieved 2010-05-29.
- ^ Kiang, Nancy Y.; Segura, Antígona; Tinetti, Giovanna; Jee, Govind; Blankenship, Robert E.; Cohen, Martin; Siefert, Janet; Crisp, David; Meadows, Victoria S. (2007-04-03). "Spectral signatures of photosynthesis. II. Coevolution with other stars and the atmosphere on extrasolar worlds". Astrobiology (Mary Ann Liebert, Inc.) 7 (1): 252–274. arXiv:astro-ph/0701391. Bibcode 2007AsBio...7..252K. doi:10.1089/ast.2006.0108. PMID 17407410. http://www.liebertonline.com/doi/abs/10.1089/ast.2006.0108. Retrieved 2010-12-03.
- ^ Jones, Barrie W. (2008). The Search for Life Continued: Planets Around Other Stars. Chichester, UK: Praxis Publishing. p. 68. ISBN 13: 978-0-387-76557-0.
- ^ "Science, "The Rise of Oxygen over the Past 205 Million Years and the Evolution of Large Placental Mammals" (30 September 2005)". Sciencemag.org. 2005-09-30. doi:10.1126/science.1116047. http://www.sciencemag.org/cgi/content/abstract/309/5744/2202. Retrieved 2010-05-29.
- ^ "Christmas in Yellowstone". Pbs.org. http://www.pbs.org/wnet/nature/yellowstone/hibernate.html. Retrieved 2010-05-29.
- ^ Main and Bentley, Ecology, "Water Relations of Australian Burrowing Frogs and Tree Frogs" (1964)
- ^ "Physicists Discover Inorganic Dust With Lifelike Qualities". Science Daily. 2007-08-15. http://www.sciencedaily.com/releases/2007/08/070814150630.htm.
- ^ a b Tsytovich, V N; G E Morfill, V E Fortov, N G Gusein-Zade, B A Klumov and S V Vladimirov (14 August 2007). "From plasma crystals and helical structures towards inorganic living matter". New J. Phys. 9 (263): 263. Bibcode 2007NJPh....9..263T. doi:10.1088/1367-2630/9/8/263. http://www.iop.org/EJ/abstract/1367-2630/9/8/263.
- ^ "Schlock Mercenary archives – Tuesday, September 5, 2006". Schlockmercenary.com. http://www.schlockmercenary.com/d/20060905.html. Retrieved 2010-05-29.
- ^ SciFi.com StarTrek Syfy[dead link]
- ^ "Excalbian. Memory Alpha". Memory-alpha.org. 2009-09-16. http://memory-alpha.org/en/wiki/Excalbian. Retrieved 2010-05-29.
- ^ Space Slug in the Star Wars Databank
- ^ Exogorth on Wookieepedia: a Star Wars Wiki
- ^ Mynock in the Star Wars Databank
- ^ Mynock on Wookieepedia: a Star Wars Wiki
- ^ Energy spider on Wookieepedia: a Star Wars Wiki
- ^ "Energy Spider. The Completely Unofficial Star Wars Encyclopedia". Cuswe.org. http://www.cuswe.org/newdescr.asp?search=7930. Retrieved 2010-05-29.
Further reading
- W. Bains (2004). "Many Chemistries Could Be Used to Build Living Systems". Astrobiology 4 (2): 137–167. Bibcode 2004AsBio...4..137B. doi:10.1089/153110704323175124. PMID 15253836. http://www.liebertonline.com/doi/abs/10.1089/153110704323175124.
External links
Categories:- Astrobiology
- Extraterrestrial life
- Science fiction themes
Wikimedia Foundation. 2010.