- Trinitromethane
-
Trinitromethane[1] 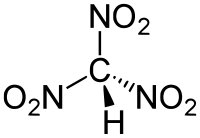 TrinitromethaneOther namesNitroform
TrinitromethaneOther namesNitroformIdentifiers CAS number 517-25-9 
ChemSpider 10157 
EC-number 208-236-8 Jmol-3D images Image 1 - C([N+](=O)[O-])([N+](=O)[O-])[N+](=O)[O-]
Properties Molecular formula CHN3O6 Molar mass 151.04 g/mol Appearance Pale yellow crystals Density 1.469 g/cm3 Melting point 15 °C, 288 K, 59 °F
Solubility in water 44g/100ml at 20°C Acidity (pKa) 0.25 (see text) Hazards Main hazards Oxidant, Explosive (esp. in contact with metals), Corrosive. NFPA 704 Related compounds Related compounds Hexanitroethane
Octanitropentane
Tetranitromethane (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Trinitromethane, also referred to as nitroform, is a nitroalkane and oxidizer with chemical formula HC(NO2)3. It was first obtained as the ammonium salt by Shiskov in 1857. In 1900, it was discovered that nitroform can be produced by the reaction of acetylene with anhydrous nitric acid. This method went on to become the industrial process of choice during the 20th century. In the laboratory, nitroform can also be produced by hydrolysis of tetranitromethane under mild basic conditions.[2]
Acidity
Trinitromethane exists in two forms, a colorless molecular form, HC(NO2)3, and an intensely yellow anion, (NO2)3C-. Nitroform is highly soluble in water, and the stable solution is yellow due to the presence of the anion. The pKa of trinitromethane has been measured at 0.17 ± 0.02 at 20°C, which is remarkably acidic for a methane derivative.[3] There is some evidence that the anion (which obeys the 4n+2 Hückel rule) is aromatic.[4]
Nitroform salts
Trinitromethane forms a series of bright yellow ionic salts. Many of these salts tend to be unstable and can be easily detonated by heat or impact. Because aqueous solutions of trinitromethane are quite acidic, this poses a handling danger similar to that of picric acid due to corrosion of iron or aluminum surfaces.
The potassium salt of nitroform, KC(NO2)3 is a lemon yellow crystalline solid that decomposes slowly at room temperatures and explodes above 95 °C. The ammonium salt is somewhat more stable, and deflagrates or explodes above 200 °C. The hydrazine salt, hydrazinium nitroformate is thermally stable to above 125 °C and is being investigated as an ecologically friendly oxidizer for use in solid fuels for rockets.
References
- ^ Budavari, Susan, ed. (1996), The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (12th ed.), Merck, ISBN 0911910123, 9859.
- ^ Gakh, A. A.; Bryan, J. C.; Burnett, M. N.; Bonnesen, P. V. (2000), "Synthesis and structural analysis of some trinitromethanide salts", J. Mol. Struct. 520 (1-3): 221–28, doi:10.1016/S0022-2860(99)00333-6.
- ^ Novikov, S. S.; Slovetskii, V. I.; Shevelev, S. A.; Fainzilberg, A. A. (1962), "Spectrophotometric Determination of the Dissociation Constants of Aliphatic Nitro Compounds", Russ. Chem. Bull. 11 (4): 552–59, doi:10.1007/BF00904751.
- ^ Cioslowski, Jerzy; Mixon, Stacey T.; Fleischmann, Eugene D. (1991), "Electronic structures of trifluoro-, tricyano-, and trinitromethane and their conjugate bases", J. Am. Chem. Soc. 113 (13): 4751–55, doi:10.1021/ja00013a007.
Categories:- Nitromethanes
Wikimedia Foundation. 2010.

