- Oxocarbon
-
An oxocarbon or oxide of carbon is an inorganic compound consisting only of carbon and oxygen.[1][2]
The simplest and most common oxocarbons are carbon monoxide (CO) and carbon dioxide (CO2). Many other stable or metastable oxides of carbon are known, but they are rarely encountered, such as carbon suboxide (C3O2 or O=C=C=C=O) and mellitic anhydride (C12O9).



CO
Carbon
monoxideCO2
Carbon
dioxideC3O2
Carbon
suboxideC12O9
Mellitic
anhydrideWhile textbooks will often list only the first three, and rarely the fourth, a large number of other oxides are known today, most of them synthesized since the 1960s. Some of these new oxides are stable at room temperature. Some are metastable or stable only at very low temperatures, but decompose to simpler oxocarbons when warmed. Many are inherently unstable and can be observed only momentarily as intermediates in chemical reactions or are so reactive that they can exist only in the gas phase or under matrix isolation conditions.
The inventory of oxocarbons appears to be steadily growing. The existence of graphene oxide and of other stable polymeric carbon oxides with unbounded molecular structures[3][4] suggests that many more remain to be discovered.
Contents
Overview
Carbon dioxide (CO2) occurs widely in nature, and was incidentally produced by humans since pre-historical times, by the combustion of carbon-containing substances and fermentation of foods such as beer and bread. It was gradually recognized as a chemical substance, formerly called spiritus sylvestre ("forest spirit") or "fixed air", by various chemists in the 17th and 18th centuries.
Carbon monoxide may occur in combustion, too, and was used (though not recognized) since antiquity for the smelting of iron from its ores. Like the dioxide, it was described and studied in the West by various alchemists and chemists since the Middle Ages. Its true composition was discovered by William Cruikshank in 1800.
Carbon suboxide was discovered by Brodie in 1873, by passing electric current through carbon dioxide.[5]
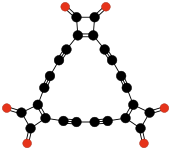
The fourth "classical" oxide, mellitic anhydride (C12O9), was apparently obtained by Liebig and Wöhler in 1830 in their study of mellite ("honeystone"), but was characterized only in 1913, by Meyer and Steiner.[6][7][8]
Brodie also discovered in 1859 a sixth compound called graphite oxide, consisting of carbon and oxygen in ratios varying between 2:1 and 3:1; but the nature and molecular structure of this substance remained unknown until a few years ago, when it was renamed graphene oxide and became a topic of research in nanotechnology.[3]
Notable examples of unstable or metastable oxides that were detected only in extreme situations are dicarbon monoxide radical (:C=C=O), carbon trioxide (CO3),[9], carbon tetroxide (CO4),[10][11] and 1,2-dioxetanedione (C2O4).[12][13] Some of these reactive carbon oxides were detected within molecular clouds in the interstellar medium by rotational spectroscopy.[14]
Many hypothetical oxocarbons have been studied by theoretical methods but have yet to be detected. Examples include oxalic anhydride (C2O3 or O=(C2O)=O), ethylene dione (C2O2 or O=C=C=O) [15] and other linear or cyclic polymers of carbon monoxide (-CO-)n (polyketones),[16] and linear or cyclic polymers of carbon dioxide (-CO2-)n, such as the dimer 1,3-dioxetanedione (C2O4)[17] and the trimer 1,3,5-trioxanetrione (C3O6).[17][18]



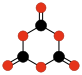

C2O3
Oxalic
anhydrideC2O4
1,2-Dioxetane-
dioneC2O4
1,3-Dioxetane-
dioneC3O6
1,3,5-Trioxane-
trioneC2O2
Ethylene
dioneGeneral structure
Normally carbon is tetravalent while oxygen is divalent, and in most oxocarbons (as in most other carbon compounds) each carbon atom may be bound to four other atoms, while oxygen may be bound to at most two. Moreover, while carbon can connect to other carbons to form arbitrarily large chains or networks, chains of three or more oxygens are rarely if ever observed. Thus the known electrically neutral oxocarbons generally consist of one or more carbon skeletons (including cyclic and aromatic structures) connected and terminated by oxide (-O-, =O) or peroxide (-O-O-) groups.
Carbon atoms with unsatisfied bonds are found in some oxides, such as the diradical C2O or :C=C=O; but these compounds are generally too reactive to be isolated in bulk.[19] Loss or gain of electrons can result in monovalent negative oxygen (-O−), trivalent positive oxygen (≡O+), or trivalent negative carbon (≡C−). The last two are found in carbon monoxide, −C≡O+.[20] Negative oxygen occurs in most oxocarbon anions.
Linear carbon dioxides
One family of carbon oxides has the general formula CnO2, or O=(C=)nO — namely, a linear chain of carbon atoms, capped by oxygen atoms at both ends. The first members are
- CO2 or O=C=O, the well-known carbon dioxide.
- C2O2 or O=C=C=O, the extremely unstable ethylene dione.[15]
- C3O2 or O=C=C=C=O, the metastable carbon suboxide or tricarbon dioxide.
- C4O2 or O=C=C=C=C=O, tetracarbon dioxide or 1,2,3-Butatriene-1,4-dione[21]
- C5O2 or O=C=C=C=C=C=O, pentacarbon dioxide,[22] stable in solution at room temp. and pure up to -90°C.[23]
Some higher member of this family have been detected in trace amounts in low-pressure gas phase and/or cryogenic matrix experiments, specifically for n = 7[23]:p.97 and n = 17, 19, and 21.[24]:p.95
Linear carbon monoxides
Another family of oxocarbons are the linear carbon monoxides CnO. The first member, ordinary carbon monoxide CO, seems to be the only one that is stable in the pure state at room temperature. Photolysis of the linear carbon dioxides in a cryogenic matrix leads to loss of CO, resulting in detectable amounts of even-numbered monoxides such as C2O, C4O,[19] and C6O.[23] The members up to n=9 have also been obtained by electrical discharge on gasous C3O2 diluted in argon.[25] The first three members have been detected in interstellar space.[25]
When n is even, the molecules are believed to be in the triplet (cumulene-like) state, with the atoms connected by double bonds and an unfilled orbital in the first carbon — as in :C=C=O, :C=C=C=C=O, and, in general, :(C=)n=O. When n is odd, the triplet structure is believed to resonate with a singlet (acetylene-type) polar state with a negative charge on the carbon end and a positive one on the oxygen end, as in −C≡C-C≡O+, −C≡C-C≡C-C≡O+, and, in general, −(C≡C-)n/2C≡O+.[25] Carbon monoxide itself follows this pattern: its predominant form is believed to be −C≡O+.[20]
Radialene-type cyclic polyketones
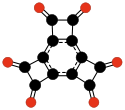
Another family of oxocarbons that has attracted special attention are the cyclic radialene-type oxocarbons CnOn or (CO)n.[26] They can be regarded as cyclic polymers of carbon monoxide, or n-fold ketones of n-carbon cycloalkanes. Carbon monoxide itself (CO) can be regarded as the first member. Theoretical studies indicate that ethylene dione (C2O2 or O=C=C=O) and cyclopropanetrione C3O3 do not exist.[15][16]. The next three members — C4O4, C5O5, and C6O6 — are theoretically possible, but are expected to be quite unstable,[16] and so far they have been synthesized only in trace amounts.[27][28]

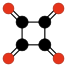
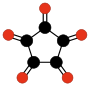
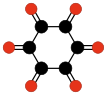
(CO)3
Cyclopropane-
trione(CO)4
Cyclobutane-
tetrone(CO)5
Cyclopentane-
pentone(CO)6
Cyclohexane
hexoneOn the other hand, the anions of these oxocarbons are quite stable, and some of them have been known since the 19th century.[26] They are
- C2O22−, acetylenediolate (Weiss and Büchner, 1963),[29]
- C3O32−, deltate (Eggerding and West, 1976),[30][31]
- C4O42−, squarate (Cohen and others, 1959),[32]
- C5O52−, croconate (Gmelin, 1825),[33] and
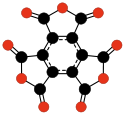
- C6O62−, rhodizonate (Heller, 1837).[34][35]
The cyclic oxide C6O6 also forms the stable anions of tetrahydroxybenzoquinone (C6O64−) and hexahydroxybenzene (C6O66−),[36] The aromaticity of these anions has been studied using theoretical methods.[37][38]
New oxides
Many new stable or metastable oxides have been synthesized since the 1960s, such as:
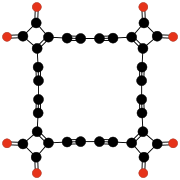
- C10O8, benzoquinonetetracarboxylic dianhydride (Hammond, 1963).[39]
- C6O6, ethylenetetracarboxylic dianhydride, a stable isomer of cyclohexanehexone (Sauer and others, 1967).[40]
- C12O12 or C6(C2O4)3, hexahydroxybenzene trisoxalate (Verter and Dominic, 1967); stable as a tetrahydrofuran solvate.[41]
- C10O10 or C6O2(C2O4)2, tetrahydroxy-1,4-benzoquinone bisoxalate (Verter and others, 1968); stable as a tetrahydrofuran solvate.[42]
- C8O8 or C6O2(CO3)2, tetrahydroxy-1,4-benzoquinone biscarbonate (Nallaiah, 1984); decomposes at about 45–53°C.[43]
- C9O9 or C6(CO3)3, hexahydroxybenzene triscarbonate (Nallaiah, 1984); decomposes at about 45–53°C.[43]
- C24O6, a cyclic trimer of the biradical 3,4-dialkynyl-3-cyclobutene1,2-dione -C≡C-(C4O2)-C≡C- (Rubin and others, 1990);[44]
- C32O8, a tetramer of 3,4-dialkynyl-3-cyclobutene1,2-dione (Rubin and others, 1990);[44]
- C4O6, dioxane tetraketone or dimeric oxalic anhydride (Strazzolini and others, 1998); stable in Et2O at −30°C, decomposes at 0°C.[45]
- C12O6, hexaoxotricyclobutabenzene (Hamura and others, 2006)[46][47]
Many relatives of these oxides have been investigated theoretically, and some are expected to be stable, such as other carbonate and oxalate esters of tetrahydroxy-1,2-benzoquinone and of the rhodizonic, croconic, squaric, and deltic acids.[16]
Polymeric carbon oxides
Carbon suboxide spontaneously polymerizes at room temperature into a carbon-oxygen polymer, with 3:2 carbon:oxygen atomic ratio. The polymer is believed to be a linear chain of fused six-membered lactone rings, with a continuous carbon backbone of alternating single and double bonds. Physical measurements indicate that the mean number of units per molecule is about 5–6, depending on the formation temperature.[4][48]

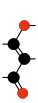
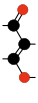

Terminating and repeating units of polymeric C3O2.[4] 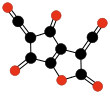
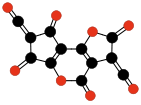
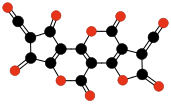
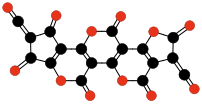
Oligomers of C3O2 with 3 to 6 units.[4] Carbon monoxide compressed to 5 GPa in a diamond anvil cell yields a somewhat similar reddish polymer with a slightly higher oxygen content, which is metastable at room conditions. It is believed that CO disproportionates in the cell to a mixture of CO2 and C3O2; the latter forms a polymer similar to the one described above (but with a more irregular structure), that traps some of the CO2 in its matrix.[49][50].
Another carbon-oxygen polymer, with C:O ratio 5:1 or higher, is the classical graphite oxide[3] and its single-sheet version graphene oxide.
See also
- Oxocarbon anion
- Pseudo-oxocarbon anion
References
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (1995) "Oxocarbons".
- ^ R. West, editor (1980), Oxocarbons. Academic Press, New York.
- ^ a b c William S. Hummers Jr., and Richard E. Offeman (1958), "Preparation of Graphitic Oxide". J. Am. Chem. Soc., 1958, 80 (6), 1339-1339 doi:10.1021/ja01539a017
- ^ a b c d A. W. Snow, H. Haubenstock, N.-L. Yang (1978): "Poly(carbon suboxide). Characterization, Polymerization, and Radical Structure". Macromolecules, 11 (1), pp 77–86. doi:10.1021/ma60061a015
- ^ Brodie B. C. (1873). "Note on the Synthesis of Marsh-Gas and Formic Acid, and on the Electric Decomposition of Carbonic Oxide". Proceedings of the Royal Society (London) 21 (139–147): 245–247. doi:10.1098/rspl.1872.0052. JSTOR 113037.
- ^ J. Liebig, F. Wöhler (1830), Ueber die Zusammensetzung der Honigsteinsäure Poggendorfs Annalen der Physik und Chemie, vol. 94, Issue 2, pp.161–164. Online version accessed on 2009-07-08.
- ^ Meyer H, Steiner K (1913.). "Über ein neues Kohlenoxyd C12O9 (A new carbon oxide C12O9)". Berichte der Deutschen Chemischen Gesellschaft 46: 813–815. doi:10.1002/cber.191304601105.
- ^ Bugge (1914), Chemie: En neues Kohenoxyd. Review of Meyer and Steiner's discovery of C12O9. Naturwissenschaftliche Wochenschrift, volume 13/29, issue 12, 22 March 1914, p. 188. Online version accessed on 2009-07-09.
- ^ DeMore W. B., Jacobsen C. W. (1969). "Formation of carbon trioxide in the photolysis of ozone in liquid carbon dioxide". Journal of Physical Chemistry 73 (9): 2935–2938. doi:10.1021/j100843a026.
- ^ Laurence Y. Yeung, Mitchio Okumura, Jeffrey T. Paci, George C. Schatz, Jianming Zhang and Timothy K. Minton (2009), Hyperthermal O-Atom Exchange Reaction O2 + CO2 through a CO4 Intermediate. J. of the American Chemical Society, volume 131, issue 39, pages 13940–13942. doi:10.1021/ja903944k
- ^ Corey S. Jamieson, Alexander M. Mebel, Ralf I. Kaiser (2007). "Novel detection of the C-2v isomer of carbon tetraoxide (CO4)". Chemical Physics Letters 440 (1–3): 105–109. doi:10.1016/j.cplett.2007.04.043.
- ^ Herman F. Cordes, Herbert P. Richter, Carl A. Heller(1969), Mass spectrometric evidence for the existence of 1,2-dioxetanedione (carbon dioxide dimer). Chemiluminescent intermediate. J. Am. Chem. Soc., 1969, 91 (25), p 7209. doi:10.1021/ja01053a065
- ^ Richard Bos, Neil W. Barnett, Gail A. Dyson, Kieran F. Lim, Richard A. Russell and Simon P. Watson (2003), Studies on the mechanism of the peroxyoxalate chemiluminescence reaction: Part 1. Confirmation of 1,2-dioxetanedione as an intermediate using 13C nuclear magnetic resonance spectroscopy. Analytica Chimica Acta, Volume 502, Issue 2, 30 January 2004, Pages 141-147. doi:10.1016/j.aca.2003.10.014
- ^ H. M. Pickett E. A. Cohen B. J. Drouin J. C. Pearson (2003), Submillimeter, Millimeter, and Microwave Spectral Line Catalog. NASA/JPL, Online verstion accessed on 2009-07-11.
- ^ a b c Detlef Schröder; Christoph Heinemann, Helmut Schwarz, Jeremy N. Harvey, Suresh Dua, Stephen J. Blanksby, John H. Bowie (1998). "Ethylenedione: An Intrinsically Short-Lived Molecule". Chemistry - A European Journal 4 (12): 2550–2557. doi:10.1002/(SICI)1521-3765(19981204)4:12<2550::AID-CHEM2550>3.0.CO;2-E.
- ^ a b c d Haijun Jiao and Hai-Shun Wu (2003), Are Neutral Oxocarbons Stable? J. Org. Chem., volume 68, 1475-1479. doi:10.1021/jo026243m
- ^ a b Errol Lewars(1996), Polymers and oligomers of carbon dioxide: ab initio and semiempirical calculations. Journal of Molecular Structure: THEOCHEM, Volume 363, Number 1, pp. 1–15
- ^ Shirel Matthew L., Pulay Peter (1999). "Stability of Novel Oxo- and Chloro-Substituted Trioxanes". J. Am. Chem. Soc 121 (37): 8544–8548. doi:10.1021/ja984451j.
- ^ a b Günter Maier and Hans Peter Reisenauer (2001), Carbenes in Matrices: Specrospcopy, Structure, and Photochemical Behavior. In Udo H. Brinker (ed.), Advances in carbene chemistry, page 135. Elsevier, 332 pages. ISBN 0444508929, 9780444508928
- ^ a b W. Kutzelnigg (2002). Einführung in die Theoretische Chemie. Wiley-VCH. ISBN 3-527-30609-9.
- ^ Günther Maier, Hans Peter Reisenauer, Heinz Balli, Willy Brandt, Rudolf Janoschek (1990): "C4O2 (1,2,3-Butatriene-1,4-dione), the First Dioxide of Carbon with an Even Number of C Atoms". Angewandte Chemie (International Edition in English), volume 29, issue 8 , Pages 905–908.
- ^ Günther Maier, Hans Peter Reisenauer, Ulrich Schäfer, and Heinz Balli (1988). "C5O2 (1,2,3,4-Pentatetraene-1,5-dione), a New Oxide of Carbon". Angewandte Chemie International Edition in English 27 (4): 566–568. doi:10.1002/anie.198805661.
- ^ a b c Frank W. Eastwood (1997), Gas Phase Pyrolytic Methods for the Preparation of Carbon-Hydrogen and Carbon-Hydrogen-Oxygen Compounds.. In Yannick ValléeGas Phase Reactions in Organic Synthesis.CRC Press; ISBN 9056990810, 9789056990817
- ^ Roman Reusch (2005), Absorptionsspektroskopie von langen Kohlenstoff-Kettenmolekülen und deren Oxide in kryogenen Matrizen. Thesis, Ruprecht-Karls-Universität Heidelberg (in German)
- ^ a b c Ogata Teruhiko, Tatamitani Yoshio (2008). "The Simplest Linear-Carbon-Chain Growth by Atomic-Carbon Addition and Ring Opening Reactions". J. Phys. Chem. A 112 (43): 10713–10715. doi:10.1021/jp806725s. PMID 18834097.
- ^ a b Gunther Seitz, Peter Imming (1992). "Oxocarbons and pseudooxocarbons". Chem. Rev. 92 (6): 1227–1260. doi:10.1021/cr00014a004.
- ^ Detlef Schröder,; Helmut Schwarz, Suresh Dua, Stephen J. Blanksby and John H. Bowie (May 1999). "Mass spectrometric studies of the oxocarbons CnOn (n = 3–6)". International Journal of Mass Spectrometry 188 (1–2): 17–25. doi:10.1016/S1387-3806(98)14208-2.
- ^ Richard B. Wyrwas and Caroline Chick Jarrold (2006), Production of C6O6- from Oligomerization of CO on Molybdenum Anions. J. Am. Chem. Soc. volume 128 issue 42, pages 13688–13689. doi:10.1021/ja0643927
- ^ Werner Büchner, E. Weiss (1963) Zur Kenntnis der sogenannten «Alkalicarbonyle» I Die Kristallstruktur des Kalium-acetylendiolats, KOC≡COK. Helvetica Chimica Acta, Volume 46 Issue 4, Pages 1121–1127. doi:10.1002/hlca.19630460404
- ^ David Eggerding and Robert West (1976), Synthesis and Properties of Deltic Acid (Dihydroxycyclopropenone) and the Deltate Ion J. American Chemical Society, volume 98, p, 3641–3644. doi:10.1021/ja00428a043
- ^ Eggerding David, West Robert (1975). "Synthesis of Dihydroxycyclopropenone (Deltic Acid)". J. American Chemical Society 97 (1): 207–208. doi:10.1021/ja00834a047.
- ^ Cohen Sidney, Lacher John R., Park Joseph D. (1959). "Diketocyclobutanediol". J. American Chemical Society 81 (13): 3480. doi:10.1021/ja01522a083.
- ^ Leopold Gmelin (1825), Ueber einige merkwürdige, bei der Darstellung des Kaliums nach der Brunner'schen Methode, erhaltene Substanzen. Poggendorfs Annalen der Physik und Chemie, volume 4, p. 31. Online version accessed on 2009-07-08.
- ^ Johann Florian Heller (1837), Die Rhodizonsäure, eine aus den Produkten der Kaliumbereitung gewonnene neue Säure, und ihre chemischen Verhältnisse, Justus Liebigs Annalen der Pharmacie, volume 24, issue 1, pp. 1–16. Online version accessed on 2009-07-08.
- ^ Carl Löwig (1839), Chemie der organischen Verbindungen. F. Schultess, Zürich.
- ^ Haiyan Chen, Michel Armand, Matthieu Courty, Meng Jiang, Clare P. Grey, Franck Dolhem, Jean-Marie Tarascon, and Philippe Poizot (2009), Lithium Salt of Tetrahydroxybenzoquinone: Toward the Development of a Sustainable Li-Ion Battery J. Am. Chem. Soc., 131 (25), pp. 8984–8988 doi:10.1021/ja9024897
- ^ R. West, J. Niu (1969, Non-benzenoid aromatics. Vol. 1. Edited by J. Snyder. Academic Press New York.
- ^ Schleyer, P. v. R.; Najafian, K.; Kiran, B.; Jiao, H. (2000). "Are Oxocarbon Dianions Aromatic?". J. Org. Chem. 65 (2): 426–431. doi:10.1021/jo991267n. PMID 10813951.
- ^ Hammond P. R. (1963). "1,4-Benzoquinone Tetracarboxylic Acid Dianhydride, C10O8: A Strong Acceptor". Science 142 (3591): 502. doi:10.1126/science.142.3591.502.
- ^ Jürgen Sauer, Barbara Schröder, Richard Wiemer (1967), Eine Studie der Diels-Alder-Reaktion, VI. Kinetischer Nachweis des Moleküls C6O6 (Dianhydrid der Äthylentetracarbonsäure). Chemische Berichte Volume 100 Issue 1, Pages 306-314 doi:10.1002/cber.19671000135
- ^ H. S. Verter, R. Dominic (1967), A new carbon oxide: synthesis of hexahydroxybenzene tris oxalate. Tetrahedron, Volume 23, Issue 10, , Pages 3863-3864 doi:10.1016/S0040-4020(01)97894-9
- ^ H. S. Verter, H. Porter, and R. Dominic (Verter, Porter and Dominic, 1968), A new carbon oxide: synthesis of tetrahydroxybenzoquinone bisoxalate. Chemical Communications (London), p. 973b–974. doi:10.1039/C1968000973b
- ^ a b C. Nallaiah (1984), Synthesis of tetrahydroxy-1,4-benzoquinone biscarbonate and hexahydroxybenzene triscarbonate - new organic carbon oxidesTetrahedron, Volume 40, Issue 23, 1984, Pages 4897-4900 doi:10.1016/S0040-4020(01)91324-9
- ^ a b Yves Rubin, Carolyn B. Knobler, and Francois Diederich (1990). "Precursors to the cyclo[n]carbons: from 3,4-dialkynyl-3-cyclobutene-1,2-diones and 3,4-dialkynyl-3-cyclobutene-1,2-diols to cyclobutenodehydroannulenes and higher oxides of carbon". J. Am. Chem. Soc. 112 (4): 1607–1617. doi:10.1021/ja00160a047.
- ^ Paolo Strazzolini, Alberto Gambi, Angelo G. Giumanini and Hrvoj Vancik (1998). "The reaction between ethanedioyl (oxalyl) dihalides and Ag2C2O4: a route to Staudinger’s elusive ethanedioic (oxalic) acid anhydride". J. Chem. Soc., Perkin Trans. 1 (16): 2553–2558. doi:10.1039/a803430c.
- ^ T. Hamura, Y. Ibusuki, H. Uekusa, T. Matsumoto, J. S. Siegel, K. K. Baldridge, K. Suzuki (2006). Dodecamethoxy- and Hexaoxotricyclobutabenzene: Synthesis and Characterization. J. Am. Chem. Soc. 128, 10032–10033. doi:10.1021/ja064063e
- ^ Holger Butenschön (2007). "A new oxocarbon C12O6 via highly strained benzyne intermediates". Angew Chem Int Ed Engl 46 (22): 4012–4014. doi:10.1002/anie.200700926. PMID 17508349.
- ^ B. D. Kybett, G. K. Johnson, C. K. Barker, and J. L. Margrave (1965), "The Heats of Formation and Polymerization of Carbon Suboxide". J. Phys. Chem., 69 (10), 3603–3606. doi:10.1021/j100894a060
- ^ A. I. Katz, D. Schiferl, and R. L. Mills (1984), New phases and chemical reactions in solid carbon monoxide under pressure. J. Physical Chemistry, volume 88 issue 15, 3176–3179. doi:10.1021/j150659a007
- ^ W. J. Evans, M. J. Lipp, C.-S. Yoo, H. Cynn, J. L. Herberg, R. S. Maxwell, and M. F. Nicol (2006), Pressure-Induced Polymerization of Carbon Monoxide: Disproportionation and Synthesis of an Energetic Lactonic Polymer. Chemistry of Materials, vol. 18, 2520–2531. doi:10.1021/cm0524446
Oxocarbons Common oxides Exotic oxides Compounds derived from oxides Inorganic compounds of carbon Oxides and related Other ionic compounds Carbides [:C≡C:]2– · Cyanides [:C≡N:]– · Cyanates [:O-C≡N:]– · Thiocyanates [:S-C≡N:]– · Fulminates [:C≡N-O:]– · Thiofulminates [:C≡N-S:]–Categories:- Oxocarbons
Wikimedia Foundation. 2010.