- Dicarbonate
-
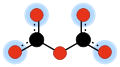
In organic chemistry, a dicarbonate is a compound containing the divalent [-O-(C=O)-O-(C=O)-O-] or C2O52• functional group, which consists of two carbonate groups sharing an oxygen atom. These compounds can be viewed as double esters of a hypothetical dicarbonic acid, H2C2O5 or HO-(C=O)-O-(C=O)-OH. Two important examples are dimethyl dicarbonate H3C-C2O5-CH3 and di-tert-butyl dicarbonate (H3C-)3C-C2O5-C(-CH3)3.
In inorganic chemistry, dicarbonate is the divalent anion C2O2−
5 or [O-(C=O)-O-(C=O)-O]2−, or any salt containing the same. It is one of the oxocarbon anions, consisting solely of oxygen and carbon. Dicarbonate salts are apparently unstable but may have a fleeting existence in carbonate solutions.[1]>The term "dicarbonate" is sometimes used erroneously to refer to bicarbonate, the common name of the hydrogencarbonate anion [HCO3]− or organic group [HCO3-].
See also
- Oxalate
- Peroxodicarbonate
- Tricarbonate
References
- ^ Zeller, Klaus-Peter; Schuler, Paul; Haiss, Peter (2005). "The hidden equilibrium in aqueous sodium carbonate solutions: Evidence for the formation of the dicarbonate anion". Eur. J. Inorg. Chem. 2005 (1): 168–172. doi:10.1002/ejic.200400445.
Oxocarbons Common oxides Exotic oxides Compounds derived from oxides Categories:- Oxoanions
- Esters
Wikimedia Foundation. 2010.