- Di-tert-butyl dicarbonate
-
Di-tert-butyl dicarbonate 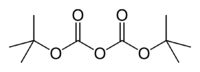
 Di-t-butyl dicarbonateOther namesdi-tert-butyl pyrocarbonate
Di-t-butyl dicarbonateOther namesdi-tert-butyl pyrocarbonate
Boc anhydride
Boc2OIdentifiers CAS number 24424-99-5 
PubChem 90495 ChemSpider 81704 
ChEBI CHEBI:48500 
Jmol-3D images Image 1 - O=C(OC(=O)OC(C)(C)C)OC(C)(C)C
Properties Molecular formula C10H18O5 Molar mass 218.25 g·mol−1 Appearance colourless solid Density 0.95 g·cm−3 Melting point 22–24 °C
Boiling point 56–57 °C (0.5 mmHg)
Solubility in water insol Solubility in other solvents most organic solvents Structure Dipole moment 0 D Hazards Main hazards toxic on inhalation T+ Related compounds Related compounds ethyl chloroformate
phosgene dicarbonate (verify) (what is:
dicarbonate (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Di-tert-butyl dicarbonate is a reagent widely used in organic synthesis.[1] This carbonate ester reacts with amines to give N-tert-butoxycarbonyl or so-called t-BOC derivatives. These derivatives do not behave as amines, which allows certain subsequent transformations to occur that would have otherwise affected the amine functional group. The t-BOC can later be removed from the amine using acids. Thus, t-BOC serves as a protective group, for instance in solid phase peptide synthesis. It is unreactive to most bases and nucleophiles, allowing for an orthogonal Fmoc protection.
Contents
Preparation
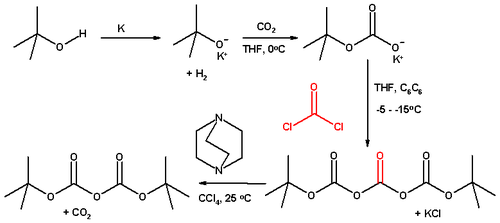
Di-tert-butyl dicarbonate is inexpensive, so it is usually purchased. Classically, this compound is prepared from tert-butanol, carbon dioxide, phosgene, using DABCO as a base:[2]
This route is currently employed commercially by manufacturers in China and India. European and Japanese companies use the reaction of sodium tert-butylate with carbon dioxide, catalysed by p-toluenesulfonic acid or methanesulfonic acid. This process involves a distillation of the crude material yielding a very pure grade.
Boc anhydride is also available as a 70% solution in toluene or THF. Since boc anhydride is a low-melting solid, having the reagent in a solution simplifies storage and handling.
Protection and deprotection of amines
The Boc group can be added to the amine under aqueous conditions using di-tert-butyl dicarbonate in the presence of a base such as sodium bicarbonate. Protection of the amine can also be accomplished in acetonitrile solution using 4-dimethylaminopyridine (DMAP) as the base.
Removal of the t-BOC in amino acids can be accomplished with strong acids such as trifluoroacetic acid neat or in dichloromethane, or with HCl in methanol.[3][4][5] A complication may be the tendency of the t-butyl cation intermediate to alkylate other nucleophiles; scavengers such as anisole or thioanisole may be used.[6][7]
Other uses
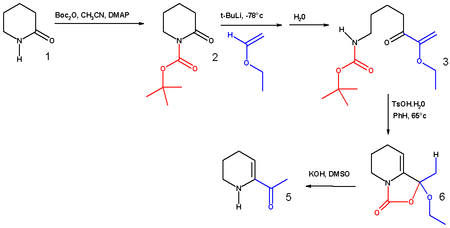
The synthesis of 6-acetyl-1,2,3,4-tetrahydropyridine, an important bread aroma compound from 2-piperidone was accomplished using t-boc anhydride.[8] (See Maillard reaction). The first step in this reaction sequence is the formation of the carbamate from the reaction of the secondary amine with boc anhydride in acetonitrile with DMAP as a base.
External links
- Protection or deprotection conditions - Synthetic protocols from organic-reaction.com
References
- ^ M. Wakselman, "Di-t-butyl Dicarbonate" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. doi:10.1002/047084289.
- ^ B. M. Pope, Y. Yamamoto, and D. S. Tarbell (1988), "Dicarbonic acid, bis(1,1-dimethylethyl) ester", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv6p0418; Coll. Vol. 6: 418
- ^ R. M. Williams, P. J. Sinclair, D. E. DeMong, D. Chen, and D. Zhai (2003), "4-Morpholinecarboxylic acid, 6-oxo-2,3-diphenyl-, 1,1-dimethylethyl ester, (2S,3R)-", Org. Synth. 80: 18, http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=v80p0018
- ^ E. A. Englund, H. N. Gopi, D. H. Appella (2004). "An Efficient Synthesis of a Probe for Protein Function: 2,3-Diaminopropionic Acid with Orthogonal Protecting Groups". Org. Lett. 6 (2): 213–215. doi:10.1021/ol0361599. PMID 14723531.
- ^ D. M. Shendage, R. Fröhlich, G. Haufe (2004). "Highly Efficient Stereoconservative Amidation and Deamidation of α-Amino Acids". Org. Lett. 6 (21): 3675–3678. doi:10.1021/ol048771l. PMID 15469321.
- ^ Lundt, Behrend F.; Johansen, Nils L.; Vølund, Aage; Markussen, Jan (1978). "Removal of t-Butyl and t-Butoxycarbonyl Protecting Groups with Trifluoroacetic acid". International Journal of Peptide and Protein Research 12 (5): 258–268. doi:10.1111/j.1399-3011.1978.tb02896.x. PMID 744685.
- ^ Andrew B. Hughes. "1. Protection Reactions". In Vommina V. Sureshbabu, Narasimhamurthy Narendra. Amino Acids, Peptides and Proteins in Organic Chemistry: Protection Reactions, Medicinal Chemistry, Combinatorial Synthesis. 4. doi:10.1002/9783527631827.ch1.
- ^ T. J. Harrison and G. R. Dake (2005). "An Expeditious, High-Yielding Construction of the Food Aroma Compounds 6-Acetyl-1,2,3,4-tetrahydropyridine and 2-Acetyl-1-pyrroline". J. Org. Chem. 70 (26): 10872–10874. doi:10.1021/jo051940a. PMID 16356012.
Further reading
- Basel, Yochai; Hassner, Alfred (2001). "Imidazole and Trifluoroethanol as Efficient and Mild Reagents for Destruction of Excess Di-tert-butyl Dicarbonate [(BOC)2O]". Synthesis 2001 (4): 0550. doi:10.1055/s-2001-12350.
Categories:- Reagents for organic chemistry
- Protecting groups
- Carbonate esters
Wikimedia Foundation. 2010.