- 4-Dimethylaminopyridine
-
4-Dimethylaminopyridine  4-DimethylaminopyridineOther namesDMAP, 4-(Dimethylamino)pyridine, N,N-Dimethyl-4-aminopyridine
4-DimethylaminopyridineOther namesDMAP, 4-(Dimethylamino)pyridine, N,N-Dimethyl-4-aminopyridineIdentifiers CAS number 1122-58-3 
PubChem 14284 ChemSpider 13646 
Jmol-3D images Image 1 - n1ccc(N(C)C)cc1
Properties Molecular formula C7H10N2 Molar mass 122.17 g/mol Melting point 110-113 °C
Boiling point 162 °C at 50 mmHg
Hazards MSDS External MSDS EU classification  T
T  C
C (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references - DMAP redirects here. For the cyanide antidote see 4-Dimethylaminophenol
4-Dimethylaminopyridine (DMAP) is a derivative of pyridine with the chemical formula (CH3)2NC5H4N. This colourless solid is a useful nucleophilic catalyst for a variety of reactions such as esterifications with anhydrides, the Baylis-Hillman reaction, hydrosilylations, tritylation, the Steglich rearrangement, Staudinger synthesis of β-lactams and many more. Chiral DMAP analogues are used in kinetic resolution experiments of mainly secondary alcohols and Evans auxiliary type amides.[1][2][3]
Contents
Preparation
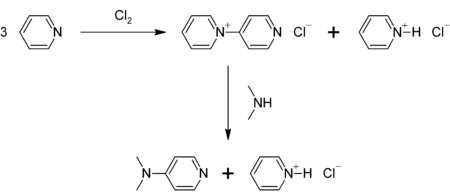
DMAP can be prepared in a two-step procedure from pyridine, which is first oxidized to 4-pyridylpyridinium cation. This cation then reacts with dimethylamine:[4]
Esterification catalyst
Main article: Steglich esterificationIn the case of esterification with acetic anhydrides the currently accepted mechanism involves three steps. First, DMAP and acetic anhydride form in a pre-equilibrium reaction a labile ion pair between the acetate and the acetylpyridinium ion. In the second step the alcohol attacks the acetyl group to form the ester. In this step the acetate counterion pulls away the proton from the alcohol while the alcohol forms a covalent bond with the acetyl group. The bond from the acetyl group to the catalyst gets cleaved to generate the catalyst and the ester. The described bond formation and breaking process runs synchronous concerted without the appearance of a tetrahedral intermediate. The acetic acid formed will then protonate the DMAP. In the last step of the catalytic cycle the auxiliary base (usually triethylamine) deprotonates the protonated DMAP, reforming the catalyst. The reaction runs through the described nucleophilic reaction pathway irrespective of the anhydride used, but the mechanism changes with the pKa value of the alcohol used. For example, the reaction runs through a base-catalyzed reaction pathway in the case of a phenol. In this case, DMAP acts as a base which deprotonates the phenol, while the formed phenolate adds to the anhydride.[5]
Safety
DMAP has a relatively high toxicity and is particularly dangerous because of its ability to be absorbed through the skin. It is also corrosive.[6]
References
- ^ Donald J Berry, Charles V Digiovanna, Stephanie S Metrick and Ramiah Murugan (2001). "Catalysis by 4-dialkylaminopyridines". Arkivoc: 201–226. http://www.arkat-usa.org/home.aspx?VIEW=MANUSCRIPT&MSID=401.
- ^ Höfle, G., Steglich, W., Vorbrüggen, H. (1978). "4-Dialkylaminopyridines as Highly Active Acylation Catalysts". Angew. Chem. Int. Ed. Engl. 17 (8): 569–583. doi:10.1002/anie.197805691.
- ^ Ryan P. Wurz (2007). "Chiral Dialkylamine Catalysts in Asymmetric Synthesis". Chem. Rev. 107 (12): 5570–5595. doi:10.1021/cr068370e. PMID 18072804. http://pubs.acs.org/doi/pdf/10.1021/cr068370e.
- ^ Shinkichi Shimizu, Nanao Watanabe, Toshiaki Kataoka, Takayuki Shoji, Nobuyuki Abe, Sinji Morishita, Hisao Ichimura "Pyridine and Pyridine Derivatives" in "Ullmann's Encyclopedia of Industrial Chemistry" 2007; Wiley-VCH, Weinheim. doi:10.1002/14356007.a22_399
- ^ S. Xu, I. Held, B. Kempf, H. Mayr, Wolfgang Steglich, H. Zipse (2005). "The DMAP-Catalyzed Acetylation of Alcohols - A Mechanistic Study (DMAP = 4-(dimethylamino)-pyridine)". Chem. Eur. J. 11 (16): 4751–4757. doi:10.1002/chem.200500398. PMID 15924289.
- ^ DMAP MSDS - Fischer Science
Further reading
- B. Neises, W. Steiglich (1990), "Esterification of Carboxylic Acids with Dicyclohexylcarbodiimide/4-Dimethylaminopyridine: tert-Butyl Ethyl Fumarate", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv7p0093; Coll. Vol. 7: 93
- I. Held, P. von den Hoff, D. S. Stephenson, H. Zipse (2008). "Domino Catalysis in the Direct Conversion of Carboxylic Acids to Esters". Adv. Synth. Cat. 11/12 (11–12): 1891–1900. doi:10.1002/adsc.200800268.
Categories:- Reagents for organic chemistry
- Amines
- Catalysts
Wikimedia Foundation. 2010.