- N,N'-Dicyclohexylcarbodiimide
-
N,N'-Dicyclohexylcarbodiimide 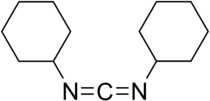
 N,N'-dicyclohexylcarbodiimideOther namesCyclohexanamine, DCC
N,N'-dicyclohexylcarbodiimideOther namesCyclohexanamine, DCCIdentifiers CAS number 538-75-0 PubChem 10868 ChemSpider 10408 
ChEBI CHEBI:53090 ChEMBL CHEMBL162598 
RTECS number FF2160000 Jmol-3D images Image 1 - N(=C=N\C1CCCCC1)\C2CCCCC2
Properties Molecular formula C13H22N2 Molar mass 206.33 g mol−1 Appearance white crystalline powder Density 1.325 g/cm3, solid Melting point 34 °C, 307 K, 93 °F
Boiling point 122°C (395 K at 6 mmHg)
Solubility in water not soluble Hazards R-phrases R22 R24 R41 R43 S-phrases S24 S26 S37/39 S45 NFPA 704 Flash point 113 °C Related compounds Related carbodiimides N,N'-diisopropylcarbodiimide,
1-ethyl-3-(3-dimethyl
aminopropyl) carbodiimide
hydrochloride (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references For other uses, see DCC (disambiguation).N,N'-Dicyclohexylcarbodiimide is an organic compound with chemical formula C13H22N2 whose primary use is to couple amino acids during artificial peptide synthesis. Under standard conditions, it exists in the form of white crystals with a heavy, sweet odor. The low melting point of this material allows it to be melted for easy handling. It is highly soluble in dichloromethane, tetrahydrofuran, acetonitrile and dimethylformamide, but insoluble in water. The compound is often abbreviated DCC.
Contents
Structure and spectroscopy
The C-N=C=N-C core of carbodiimides (N=C=N) is linear, being related to the structure of allene. Three principal resonance structures describe carbodiimides:
- RN=C=NR ↔ RN+≡C-N-R ↔ RN--C≡N+R
The N=C=N moiety gives characteristic IR spectroscopic signature at 2117 cm-1.[1] The 15N NMR spectrum shows a characteristic shift of 275.0 ppm upfield of nitric acid and the 13C NMR spectrum features a peak at about 139 ppm downfield from TMS.[2]
Preparation
Of the several syntheses of DCC, Pri-Bara et al. use palladium acetate, iodine, and oxygen to couple cyclohexyl amine and cyclohexyl isocyanide.[3] Yields of up to 67% have been achieved using this route:
- C6H11NC + C6H11NH2 + O2 → (C6H11N)2C + H2O
Tang et al. condense two isocyanates using the catalyst OP(MeNCH2CH2)3N in yields of 92%:[1]
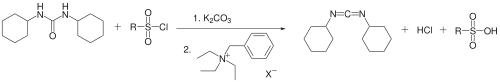
DCC has also been prepared from dicyclohexylurea using a phase transfer catalyst by Jaszay et al. The disubstituted urea, arenesulfonyl chloride, and potassium carbonate react in toluene in the presence of benzyl triethylammonium chloride to give DCC in 50% yield.[4]
Reactions
DCC is a dehydrating agent for the preparation of amides, ketones, nitriles. In these reactions, DCC hydrates to form dicyclohexylurea (DCU), a compound that is insoluble in water. DCC can also be used to invert secondary alcohols.
Moffatt oxidation
A solution of DCC and dimethyl sulfoxide (DMSO) effects the so-called Pfitzner-Moffatt oxidation. This procedure is used for the oxidation of alcohols to aldehydes and ketones. Unlike metal-mediated oxidations, the reaction conditions are sufficiently mild to avoid over-oxidation of aldehydes to carboxylic acids. Generally, three equivalents of DCC and 0.5 equivalent of proton source in DMSO and allowed to react overnight at room temperature. The reaction is quenched with acid:

Dehydration
Alcohols can also be dehydrated using DCC. This reaction proceeds by first giving the O-acylurea intermediate which is then hydrogenolyzed to produce the corresponding alkene:
- RCHOHCH2R' + (C6H11N)2C → RCH=CHR' + (C6H11NH)2CO
Inversion of secondary alcohols
Secondary alcohols can be stereochemically inverted by formation of a formyl ester followed by saponification. The secondary alcohol is mixed directly with DCC, formic acid, and a strong base such as sodium methoxide.
Esterification
Main article: Steglich esterificationA range of alcohols, including even some tertiary alcohols, can be esterified using a carboxylic acid in the presence of DCC and a catalytic amount of DMAP.[5]
DCC-promoted peptide coupling
During protein synthesis (such as Fmoc solid-state synthesizers), the N-terminus is often used as the attachement site on which the amino acid monomers are added. To enhance the electrophilicity of carboxylate group, the negatively charged oxygen must first be "activated" into a better leaving group. DCC is used for this purpose. The negatively charged oxygen will act as a nucleophile, attacking the central carbon in DCC. DCC is temporarily attached to the former carboxylate group forming a highly electrophilic intermediate, making nucleophilic attack by the terminal amino group on the growing peptide more efficient.
Safety
DCC is a potent allergen and a sensitizer, often causing skin rashes.
See also
References
- ^ a b Jiansheng Tang, Thyagarajan Mohan, John G. Verkade (1994). "Selective and Efficient Syntheses of Perhydro-1 ,3,5-triazine-2,4,6-trioneasn d Carbodiimides from Isocyanates Using ZP(MeNCH2CH2)sN Catalysts". J. Org. Chem. 59: 4931–4938. doi:10.1021/jo00096a041.
- ^ Issa Yavari, John D. Roberts (1978). "Nitrogen-15 Nuclear Magnetic Resonance Spectroscopy. Carbodiimides". J. Org. Chem. 43: 4689–4690. doi:10.1021/jo00419a001.
- ^ Ilan Pri-Bara and Jeffrey Schwartz (1997). "N,N-Dialkylcarbodiimide synthesis by palladium-catalysed coupling of amines with isonitriles". Chem Commun 4: 347. doi:10.1039/a606012i.
- ^ Zsuzsa Jaszay, Imre Petnehazy, Laszlo Toke, Bela Szajani (1987). "Preparation of Carbodiimides Using Phase-Transfer Catalysis". Synthesis 5: 520–523. doi:10.1055/s-1987-27992.
- ^ B. Neises, W. Steglich (1990), "Esterification of Carboxylic Acids with Dicyclohexylcarbodiimide/4-Dimethylaminopyridine: Tert-Butyl Ethyl Fumarate", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv7p0093; Coll. Vol. 7: 93
External links
- An excellent illustration of this mechanism can be found here: [1].
Categories:- Dehydrating agents
- Peptide coupling reagents
- Carbodiimides
Wikimedia Foundation. 2010.