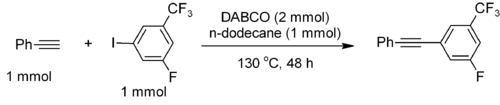- DABCO
-
DABCO 
 1,4-diazabicyclo[2.2.2]octaneOther namestriethylenediamine, TEDA
1,4-diazabicyclo[2.2.2]octaneOther namestriethylenediamine, TEDA
DABCOIdentifiers CAS number 280-57-9 
PubChem 9237 ChemSpider 8882 
Jmol-3D images Image 1
Image 2- C1CN2CCN1CC2
N12CCN(CC1)CC2
Properties Molecular formula C6H12N2 Molar mass 112.17 g/mol Appearance White crystalline powder Melting point 156 - 160 °C
Boiling point 174 °C
Solubility in water Soluble, hygroscopic Hazards[1] R-phrases R11 R22 R36 R37 R38 R52 R53 Main hazards Harmful  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references DABCO or 1,4-diazabicyclo[2.2.2]octane is a chemical compound. It is a polyurethane and Baylis-Hillman reaction catalyst, complexing ligand and Lewis base.[2] It is used to regulate the reaction rate in Flexplay time-limited DVDs by adjusting pH. Antioxidants, like DABCO, are used to improve the lifetime of dyes. This makes DABCO useful in dye lasers and in mounting samples for fluorescence microscopy (when used with glycerol and PBS).[3] DABCO can also be used to demethylate quaternary ammonium salts by heating in N,N-dimethylformamide (DMF).[4]
DABCO has been used as a catalyst for a metal-free Sonogashira coupling, with or without microwave enhancement.[5] For example, phenylacetylene couples with electron-deficient iodoarenes to furnish the Sonogashira product in 77% yield with 95% selectivity.
Dabco is a registered trademark for Air Products' catalyst product line including 1,4-diazabicyclo[2.2.2]octane.
References
- ^ "Safety data for 1,4-diazabicyclo(2.2.2)octane". Oxford University. http://physchem.ox.ac.uk/MSDS/DI/1,4-diazabicyclo(2.2.2)octane.html.
- ^ Luca Cecchi1, Francesco De Sarlo, Fabrizio Machetti (August 2006). "1,4-Diazabicyclo[2.2.2]octane (DABCO) as an Efficient Reagent for the Synthesis of Isoxazole Derivatives from Primary Nitro Compounds and Dipolarophiles: The Role of the Base". Eur. J. Org. Chem. 2006 (21): 4852–4860. doi:10.1002/ejoc.200600475.
- ^ Valnes K, Brandtzaeg P (August 1985). "Retardation of immunofluorescence fading during microscopy". J. Histochem. Cytochem. 33 (8): 755–61. PMID 3926864. http://www.jhc.org/cgi/pmidlookup?view=long&pmid=3926864.
- ^ Ho, T.L (1972). "Dealkylation of Quaternary Ammonium Salts with 1,4-Diazabicyclo[2.2.2]octane". Synthesis 1972 (12): 702. doi:10.1055/s-1972-21977.
- ^ Luque, Rafael; Duncan J Macquarrie (2009). "Efficient solvent- and metal-free Sonogashira protocol catalysed by 1,4-diazabicyclo(2.2.2) octane (DABCO)". Organic and Biomolecular Chemistry (Royal Society of Chemistry) 7 (8): 1627–1632. doi:10.1039/b821134p. PMID 19343249.
Categories:- Amines
- Nitrogen heterocycles
- C1CN2CCN1CC2
Wikimedia Foundation. 2010.