- Baylis–Hillman reaction
-
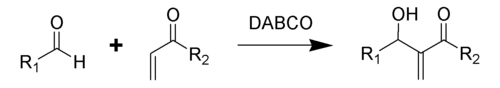
The Baylis–Hillman reaction is an organic reaction of an aldehyde and an α,β-unsaturated electron-withdrawing group catalyzed by DABCO (1,4-diazabicyclo[2.2.2]octane) to give an allylic alcohol.[1][2] This reaction is also known as the Morita–Baylis–Hillman reaction or MBH reaction.[3] It is named for the Japanese chemist Ken-ichi Morita, the British chemist Anthony B. Baylis and the German chemist Melville E. D. Hillman. The Baylis–Hillman reaction, in the present day version, is an atom-economic carbon-carbon bond formation reaction.
In addition to DABCO, additional nucleophilic amines such as DMAP and DBU as well as phosphines have been found to successfully catalyze this reaction.
Several reviews have been written. [4] [5] [6] [7] [8]
Contents
Reaction mechanism
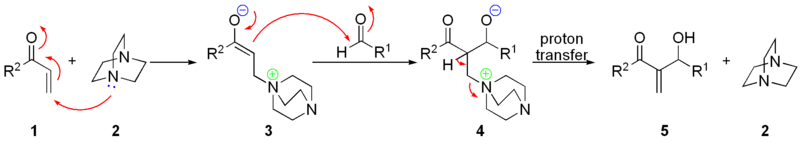
The nucleophilic addition of DABCO 2 onto the α,β-unsaturated ketone 1 gives a zwitterionic intermediate 3, which will add to the electrophilic aldehyde producing the keto-alcohol 4. Elimination of the DABCO gives the desired allylic alcohol 5.
A simple relationship exists between pKa of the base (as its conjugate acids) and the reaction rate with quinuclidine even more effective than DABCO. Protic additives like methanol, triethanolamine, formamide, and water also accelerate the reaction [9].
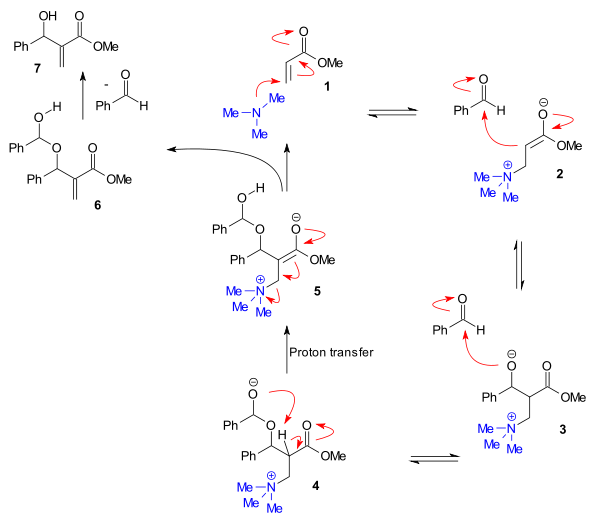
An alternative mechanism, based on extensive rate data, has been proposed for some aldehydes.[10][11][12]. This mechanism (figure below) takes into account experimentally determined second order kinetics for the aldehyde and a substantial kinetic isotope effect for the enone alpha-proton. In it a second aldehyde molecule reacts to form a hemiacetal (4) and this step is followed by a rate-determining proton transfer step to intermediate 5.
In silico experiments confirm this mechanism [13] and also explain how protic additives increase reaction rates by facilitating the proton transfer step. Several of the key intermediates can also be detected experimentally with ESI-MS [14]
A related reaction actually predating the Baylis–Hillman reaction utilising phosphines and not DABCO is the lesser known Rauhut–Currier reaction.
Scope
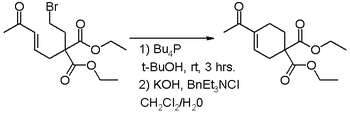
The MBH reaction in general is any reaction of electron deficient alkenes and sp2 hybridized carbon electrophiles such as aldehydes, ketones and aldimines catalyzed by a nucleophile. Under special reaction conditions the reaction is also found to extend to alkyl halides as the electrophilic reagent [15]. In this variation amine nucleophiles are unsuitable and trialkyl phosphines are used instead. Under the given reaction conditions these phosphines do not react directly with the alkyl halide. The added base in the second step of this reaction promotes the elimination reaction to the enone.
In the aza-Baylis–Hillman reaction the electrophile is an imine.[16]
Recently Namboothiri and Deb [17] developed a novel methodology for the synthesis of substituted α-hydroxyalkylated nitroalkenes by using MBH strategy under DMAP/MeCN and Imidazole/THF catalytic condition. Various activated carbonyl compounds like ethylglyoxylate, pyruvic aldehyde, trifluoromethyl pyruvate, diethylketomalonate were used as electrophiles and various aromatic, heteroaromatic, conjugated nitroalkenes were used as MBH substrates.
The Baylis–Hillman adducts and their derivatives have been extensively utilized for the generation of heterocycles and other cyclic frameworks.[18]
Limitations
The MBH reaction of phenyl vinyl ketone with benzaldehyde and DABCO in DMF is not limited to the monoadduct because the MBH adduct reacts with a second molecule of phenyl vinyl ketone in a nucleophilic conjugate addition [19].
References
- ^ Baylis, A. B.; Hillman, M. E. D. German Patent 2155113, 1972.
- ^ Ciganek, E. Org. React. 1997, 51, 201. (doi: 10.1002/0471264180.or051.02)
- ^ K. Morita, Z. Suzuki and H. Hirose, Bull. Chem. Soc. Jpn.,1968, 41, 2815.
- ^ Recent Advances in the Baylis−Hillman Reaction and Applications Deevi Basavaiah, Anumolu Jaganmohan Rao, and Tummanapalli Satyanarayana Chem. Rev., 2003, 103 (3), pp 811–892 2003 (Article) doi:10.1021/cr010043d
- ^ Masson, G., Housseman, C. and Zhu, J. (2007), The Enantioselective Morita–Baylis–Hillman Reaction and Its Aza Counterpart. Angewandte Chemie International Edition, 46: 4614–4628. doi:10.1002/anie.200604366
- ^ aza-Baylis−Hillman Reaction Valerie Declerck, Jean Martinez and Frederic Lamaty Chem. Rev., 2009, 109 (1), pp 1–48, 2009 (Review) doi:10.1021/cr068057c
- ^ Recent Contributions from the Baylis−Hillman Reaction to Organic Chemistry Deevi Basavaiah, Bhavanam Sekhara Reddy and Satpal Singh Badsara Chemical Reviews 2010 110 (9), 5447-5674 doi:10.1021/cr900291g
- ^ The Baylis–Hillman reaction: a novel concept for creativity in chemistry Deevi Basavaiah and Gorre Veeraraghavaiah Chem. Soc. Rev., 2012, Advance Article doi:10.1039/C1CS15174F
- ^ Correlation between pKa and Reactivity of Quinuclidine-Based Catalysts in the Baylis–Hillman Reaction: Discovery of Quinuclidine as Optimum Catalyst Leading to Substantial Enhancement of Scope Aggarwal, V. K.; Emme, I.; Fulford, S. Y. J. Org. Chem. (Article); 2003; 68(3); 692-700. doi:10.1021/jo026671s
- ^ Baylis–Hillman Mechanism: A New Interpretation in Aprotic Solvents Price, K. E.; Broadwater, S. J.; Jung, H. M.; McQuade, D. T.; Org. Lett., 2005, 7(1), 147-150. doi:10.1021/ol047739o
- ^ A New Interpretation of the Baylis–Hillman Mechanism Price, K. E.; Broadwater, S. J.; Walker, B. J.; McQuade, D. T. J. Org. Chem. (Article); 2005; 70(10); 3980-3987. doi:10.1021/jo050202j
- ^ Synthetic potential of the tertiary-amine-catalysed reaction of activated vinyl carbanions with aldehydes Drewes, S. E.; Roos, G. H. P.; Tetrahedron 1988, 44, 4653-4670. doi:10.1016/S0040-4020(01)86168-8
- ^ Mechanism of the Morita–Baylis–Hillman Reaction: A Computational Investigation Raphael Robiette, Varinder K Aggarwal, and Jeremy N. Harvey J. AM. CHEM. SOC. 2007, 129, 15513-15525 doi:10.1021/ja0717865
- ^ Dualistic Nature of the Mechanism of the Morita–Baylis–Hillman Reaction Probed by Electrospray Ionization Mass Spectrometry Giovanni W. Amarante, Humberto M. S. Milagre, Boniek G. Vaz, Bruno R. Vilacha Ferreira, Marcos N. Eberlin, and Fernando Coelho J. Org. Chem., 2009, 74 (8), 3031-3037 doi:10.1021/jo802578t
- ^ Unprecedented reactivity in the Morita–Baylis–Hillman reaction; intramolecular -alkylation of enones using saturated alkyl halides Marie E. Krafft, Kimberly A. Seibert, Thomas F. N. Haxell and Chitaru Hirosawa Chemical Communications, 2005, (46), 5772 - 5774 DOI: 10.1039/b512665g Abstract
- ^ Enantioselective aza-Baylis–Hillman Reaction Vasco D.B. Bonifacio, Org. Chem. Highlights, 2006, Full Article
- ^ Hydroxyalkylation of Conjugated Nitroalkenes with Activated Nonenolizable Carbonyl Compounds Indubhusan Deb,Mamta Dadwal, Shaikh M. Mobin, and Irishi N. N. NamboothiriOrganic Letters, 2006, (8), 1201 - 1204 DOI: http://pubs.acs.org/doi/full/10.1021/ol060041l]
- ^ Advances in the Baylis–Hillman Reaction-Assisted Synthesis of Cyclic Frameworks. Singh V. Batra S. Tetrahedron, "'2008"', 64(20), 4511-4574.
- ^ Different Reaction Patterns in the Baylis–Hillman Reaction of Aryl Aldehydes with Phenyl Vinyl Ketone, Phenyl Acrylate and Phenyl Thioacrylate Min Shi, Chao-Qun Li and Jian-Kang Jiang Molecules 2002, 7, 721-733 Full Article
Categories:- Addition reactions
- Carbon-carbon bond forming reactions
- Name reactions
Wikimedia Foundation. 2010.