- Smelting
-
Smelting is a form of extractive metallurgy; its main use is to produce a metal from its ore. This includes iron extraction from iron ore, and copper extraction and other base metals from their ores. Smelting uses heat and a chemical reducing agent to change the oxidation state of the metal ore; the reducing agent is commonly a source of carbon such as coke, or in earlier times charcoal. The carbon or carbon monoxide derived from it removes oxygen from the ore to leave the metal. The carbon is thus oxidized in two stages, producing first carbon monoxide and then carbon dioxide. As most ores are impure, it is often necessary to use flux, such as limestone, to remove the accompanying rock gangue as slag.
Plants for the electrolytic reduction of aluminium are also generally referred to as smelters. These do not melt aluminum oxide but instead dissolve it in aluminum fluoride. They normally use carbon electrodes, and conversion of carbon to carbon dioxide provides an important part of deoxydation energy, rest of energy being provided by electricity, but novel smelter designs use electrodes that are not consumed in the process. The end product is molten aluminum.
Contents
Process
Smelting involves more than just "melting the metal out of its ore". Most ores are a chemical compound of the metal with other elements, such as oxygen (as an oxide), sulfur (as a sulfide) or carbon and oxygen together (as a carbonate). To produce the metal, these compounds have to undergo a chemical reaction. Smelting therefore consists of using suitable reducing substances that will combine with those oxidizing elements to free the metal.
Roasting
In the case of carbonates and sulfides, a process called "roasting" drives off the unwanted carbon or sulfur, leaving an oxide, which can be directly reduced. Roasting is usually carried out in an oxidizing environment. A few practical examples:
- Malachite, a common ore of copper, is primarily copper carbonate (CuCO3). This mineral undergoes thermal decomposition to CuO and CO2 in several stages between 250°C and 350°C. The carbon dioxide is expelled into the atmosphere, leaving copper oxide which can be directly reduced to copper as shown below.
- Galena, the most common mineral of lead, is primarily lead sulfide (PbS). The sulfide is oxidized to a sulfite (PbSO3) which thermally decomposes into lead oxide and sulfur dioxide gas. (PbO and SO2) The sulfur dioxide (like the carbon dioxide in the example above) is expelled, and the lead oxide is reduced as below.
Reduction
Reduction is the final, high-temperature step in smelting. It is here that the oxide becomes the elemental metal. A reducing environment, (often provided by carbon monoxide in an air-starved furnace) pulls the final oxygen atoms from the raw metal. The required temperature varies over a very large range, both in absolute terms, and in terms of the melting point of the base metal. A few examples:
- iron oxide becomes metallic iron at roughly 1250°C, almost 300 degrees below iron's melting point of 1538°C
- mercuric oxide becomes vaporous mercury near 550°C, almost 600 degrees above mercury's melting point of -38°C
Fluxes
Fluxes are used in smelting for several purposes, chief among them catalyzing the desired reactions and chemically binding to unwanted impurities or reaction products. Calcium oxide, in the form of lime, was often used for this purpose, since it could react with the carbon dioxide sulfur dioxide produced during roasting and smelting to keep them out of the working environment.
History
Historically, the first smelting processes used carbon (in the form of charcoal) to reduce the oxides of tin (cassiterite, SnO2), copper (cuprite, CuO) and lead (Lead(II) oxide, PbO), and eventually iron (hematite, Fe2O3) In all these reactions the reducing agent was actually carbon monoxide, as the charcoal and oxides remained solid and therefore could not react directly with each other. For copper and lead, important ores were actually the sulfides, chalcocite (CuS2) and galena (PbS); these first had to be converted to oxides by roasting them in air
Silver was generally found in the form of sulfides mixed with galena; it was smelted together with lead and was afterwards separated from it by cupellation.
The primary source of mercury was the sulfide ore (cinnabar, HgS). This was roasted to produce the oxide HgO, which decomposed into oxygen and mercury, which both left the furnace as a (highly toxic) vapor, that was condensed in appropriate containers.
In the Old World, humans learned to smelt metals in prehistoric times, more than 8000 years ago. The discovery and use of the "useful" metals — copper and bronze at first, then iron a few millennia later — had an enormous impact on human society. The impact was so pervasive that scholars traditionally divide ancient history into Stone Age, Bronze Age, and Iron Age.
In the Americas, pre-Inca civilizations of the central Andes in Peru had mastered the smelting of copper and silver at least six centuries before the first Europeans arrived in the 16th century.[1]
Tin and lead
In the Old World, the first metals smelted were tin and lead. The earliest known cast lead beads were found in the Çatal Höyük site in Anatolia (Turkey), and dated from about 6500 BC, but the metal may have been known earlier.
Since the discovery happened several millennia before the invention of writing, we have no evidence about how it was made. However, tin and lead can be smelted by placing the ores in a wood fire, so it is possible that the discovery may have occurred by accident.
Although lead is a common metal, its discovery had relatively little impact in the ancient world. It is too soft to be used for weapons (except possibly as sling projectiles) or for structural elements. However, being easy to cast and shape, it came to be extensively used in the classical world of Ancient Greece and Ancient Rome for piping and storage of water. It was also used as a mortar in stone buildings, and as a writing material for commemorative plaques and curses.
Tin was much less common than lead and is only marginally harder, and had even less impact by itself.
Copper and bronze
After tin and lead, the next metal to be smelted appears to have been copper. How the discovery came about is a matter of much debate. Campfires are about 200 °C short of the temperature needed for that, so it has been conjectured that the first smelting of copper may have been achieved in pottery kilns. The earliest known instances in Europe and the Near East were found in Iran, dated about 6000 BC. The first smelted copper artifact was thought to be a mace head found in Can Hasan, Turkey and dated to 5000 BC. However, this now appears to be hammered native copper.[2] The earliest current evidence of copper smelting, dating from between 5500 BC and 5000 BC, has been found in Pločnik and Belovode, Serbia.[3][4]
The development of copper smelting in the Andes, which is believed to have occurred independently of that in the Old World, may have occurred in the same way.[1]
By combining copper with tin and/or arsenic in the right proportions one obtains bronze, an alloy which is significantly harder than copper. The first copper/arsenic bronzes date of 4200 BC from Asia Minor. The Inca bronze alloys were also of this type. Arsenic is often an impurity in copper ores, so the discovery could have been made by accident; but eventually arsenic-bearing minerals were intentionally added during smelting.[citation needed]
Copper/tin bronzes, harder and more durable, were developed around 1500 BC or 3200 BC,also in Asia Minor.[citation needed]
The discovery of copper smelting and bronze manufacture had a significant impact on the history of the Old World. The metal was hard enough to make weapons that were heavier, stronger, and more resistant to impact-related damage than their wood, bone, or stone equivalents. For several millennia, bronze was the material of choice for weapons such as swords, daggers, battle axes, and spear and arrow points, as well as protective gear such as shields, helmets, shin guards, and other body armor. Bronze also supplanted stone, wood, and organic materials in all sorts of tools and household utensils, such as chisels, saws, adzes, nails, blade shears, knives, sewing needles and pins, jugs, cooking pots and cauldrons, mirrors, horse harnesses, and much more.[citation needed] In turn, bronze tools brought about an increase in the quantity, quality, and complexity of manufactured products, from buildings to leather goods.[citation needed] Tin and copper also contributed to the establishment of trade networks spanning large areas of Europe and Asia, and had a major effect on the distribution of wealth among individuals and nations.[citation needed]
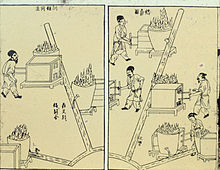 Casting bronze ding-tripods, from the Chinese Tiangong Kaiwu encyclopedia of Song Yingxing, published in 1637.
Casting bronze ding-tripods, from the Chinese Tiangong Kaiwu encyclopedia of Song Yingxing, published in 1637.
The process through which the smiths learned to produce copper/tin bronzes is once again a mystery. The first such bronzes were probably a lucky accident from tin contamination of copper ores, but by 2000 BC, we know that tin was being mined on purpose for the production of bronze. This is amazing, given that tin is a semi-rare metal, and even a rich cassiterite ore only has 5% tin. Also, it takes special skills (or special instruments) to find it and to locate the richer lodes. But, whatever steps were taken to learn about tin, these were fully understood by 2000 BC.
Early iron smelting
The earliest evidence to date for the bloomery smelting of iron is found at Tell Hammeh, Jordan (see also external link), and dates to 930 BC (C14 dating). However, based on the archaeological record of iron artifacts, it is clear that intentional reduction of iron metal from terrestrial ores (in the case of Hammeh a Haematite ore), must have started near the end of the Late Bronze Age (ca. 1600–1150 BC). Where and how iron smelting was discovered is widely debated, and remains uncertain due to the significant lack of production finds. Nevertheless, there is some consensus[citation needed] that iron technology originated in the Near East, perhaps in Eastern Anatolia.
In Ancient Egypt, somewhere between the Third Intermediate Period and 23rd Dynasty (ca. 1100–750 BC), there are indications of iron working. Significantly though, no evidence for the smelting of iron from ore has been attested to Egypt in any period. There is a further possibility of iron smelting and working in West Africa by 1200 BC.[5] In addition, very early instances of carbon steel were found to be in production around 2000 years before the present in northwest Tanzania, based on complex preheating principles. These discoveries are significant for the history of metallurgy.[6]
Most early processes in Europe and Africa involved smelting iron ore in a bloomery, where the temperature is kept low enough so that the iron does not melt. This produces a spongy mass of iron called a bloom, which then has to be consolidated with a hammer.
Later iron smelting
From the medieval period, the process of direct reduction in bloomeries began to be replaced by an indirect process. In this, a blast furnace was used to make pig iron, which then had to undergo a further process to make forgeable bar iron. Processes for the second stage include fining in a finery forge and, from the Industrial Revolution, puddling. However both processes are now obsolete, and wrought iron is now hardly made. Instead, mild steel is produced from a bessemer converter or by other means.
Base metals
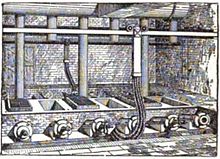 Cowles Syndicate of Ohio in Stoke-upon-Trent England, late 1880s. British Aluminium used the process of Paul Héroult about this time.[7]
Cowles Syndicate of Ohio in Stoke-upon-Trent England, late 1880s. British Aluminium used the process of Paul Héroult about this time.[7]
The ores of base metals are often sulfides. In recent centuries, reverberatory smelters (sometimes called cupolas) have been used. These keep the fuel and the charge being smelted separate. Traditionally these were used for carrying out the first step: formation of two liquids, one an oxide slag containing most of the impurity elements, and the other a sulfide matte containing the valuable metal sulfide and some impurities. Such "reverb" furnaces are today about 40 m long, 3 m high and 10 m wide. Fuel is burned at one end and the heat melts the dry sulfide concentrates (usually after partial roasting), which is fed through the openings in the roof of the furnace. The slag floats on top of the heavier matte, and is removed and discarded or recycled. The sulfide matte is then sent to the converter. However the precise details of the process will vary from one furnace to another depending on the mineralogy of the orebody from which the concentrate origninates.
More recent furnaces have been designed based upon bath smelting, top jetting lance smelting, flash smelting and blast furnaces. Some examples of bath smelters include, the noranda furnace, the teniente reactor, the vunyukov smelter and the SKS technology to name a few. Top jetting lance smelters include the mitsubishi smelting reactor. The flash smelters consist of over 50% of the worlds copper smelters. There are many more varies smelters as well, including the kievset, Ausmelt, Isasmelt, Tamano, EAF, and BF.
See also
References
- ^ a b http://www.sciencedaily.com/releases/2007/04/070423100437.htm
- ^ http://books.google.co.uk/books?id=QHAlOAAACAAJ&dq=ancient+turkey&hl=en&ei=V4M7TOnjAcH8sQaPhrzvBg&sa=X&oi=book_result&ct=result&resnum=2&ved=0CDIQ6AEwAQ
- ^ http://www.stonepages.com/news/archives/002557.html
- ^ http://www.archaeologydaily.com/news/201006274431/Belovode-site-in-Serbia-may-have-hosted-first-copper-makers.html
- ^ How Old is the Iron Age in Sub-Saharan Africa? - by Roderick J. McIntosh, Archaeological Institute of America (1999)
- ^ Peter Schmidt, Donald H. Avery. Complex Iron Smelting and Prehistoric Culture in Tanzania, Science 22 September 1978: Vol. 201. no. 4361, pp. 1085 - 1089
- ^ Minet, Adolphe (1905). The Production of Aluminum and Its Industrial Use. Leonard Waldo (translator, additions). New York, London: John Wiley and Sons, Chapman & Hall, via Google Books scan of University of Wisconsin - Madison copy. pp. 244 (Minet speaking) +116 (Héroult speaking). http://books.google.com/books?id=g14wAAAAMAAJ&pg=PA244. Retrieved 2007-10-28.
Bibliography
- Pleiner, R. (2000) Iron in Archaeology. The European Bloomery Smelters, Praha, Archeologický Ústav Av Cr.
- Veldhuijzen, H.A. (2005) Technical Ceramics in Early Iron Smelting. The Role of Ceramics in the Early First Millennium Bc Iron Production at Tell Hammeh (Az-Zarqa), Jordan. In: Prudêncio, I.Dias, I. and Waerenborgh, J.C. (Eds.) Understanding People through Their Pottery; Proceedings of the 7th European Meeting on Ancient Ceramics (Emac '03). Lisboa, Instituto Português de Arqueologia (IPA).
- Veldhuijzen, H.A. and Rehren, Th. (2006) Iron Smelting Slag Formation at Tell Hammeh (Az-Zarqa), Jordan. In: Pérez-Arantegui, J. (Ed.) Proceedings of the 34th International Symposium on Archaeometry, Zaragoza, 3–7 May 2004. Zaragoza, Institución «Fernando el Católico» (C.S.I.C.) Excma. Diputación de Zaragoza.
External links
Categories:- Dutch loanwords
- Metallurgy
- Metallurgical processes
- Steelmaking
Wikimedia Foundation. 2010.

