- Cyclohexanehexone
-
Cyclohexanehexone 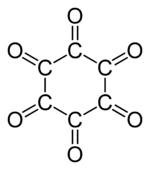 cyclohexane-1,2,3,4,5,6-hexoneOther nameshexaketocyclohexane, triquinoyl
cyclohexane-1,2,3,4,5,6-hexoneOther nameshexaketocyclohexane, triquinoylIdentifiers CAS number 527-31-1 PubChem 68240 ChemSpider 61541 
Jmol-3D images Image 1 - O=C1C(=O)C(=O)C(=O)C(=O)C1=O
Properties Molecular formula C6O6 Molar mass 168.06 g mol−1  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Cyclohexanehexone, also known as hexaketocyclohexane and triquinoyl, is a organic compound with formula C6O6, the sixfold ketone of cyclohexane. It is an oxide of carbon (an oxocarbon), indeed a hexamer of carbon monoxide.
As of May 2006, this compound had yet to be synthesized in bulk.[1][2]
Contents
Related compounds
Cyclohexanehexone can be viewed as the neutral counterpart of the rhodizonate anion C6O2−
6. The singly charged anion C6O−
6 has been detected in mass spectrometry experiments by R. Wyrwas and C. Jarrold, formed by oligomerization of carbon monoxide through the formation molybdenum carbonyls.[3]According to X-ray diffraction analysis, the reagent traded under the name "cyclohexanehexone octahydrate" or equivalent names is actually dodecahydroxycyclohexane dihydrate, a solid that decomposes at 95°C.[4][5]
In 1966, H. E. Worne of Natick Chemical Industries patented compounds with formulas C10O8 and C14O10, which can be described as the fusion of two or three molecules of C6O6, claimed to be produced by the action of ultraviolet radiation on a hot water solution of the parent compound.[6]
Triquinoyl therapy
In the late 1940s, W. Hale claimed that triquinoyl, being a trimer of W. Koch's glyoxylide, should be just as effective as the latter against "diabetes, arthritis, poliomyelitis, and even cancer".[7] Even though there is no research supporting this claim (and Koch's glyoxylide preparations were found to be just distilled water),[8] triquinoyl is still listed as an ingredient of some alternative medicine remedies.[9][10]
References
- ^ Gunther Seitz; Peter Imming (1992). "Oxocarbons and pseudooxocarbons". Chemical Reviews 92 (6): 1227–1260. doi:10.1021/cr00014a004.
- ^ Detlef Schröder,; Helmut Schwarz, Suresh Dua, Stephen J. Blanksby and John H. Bowie (1999). "Mass spectrometric studies of the oxocarbons CnOn (n = 3–6)". International Journal of Mass Spectrometry 188 (1–2): 17–25. doi:10.1016/S1387-3806(98)14208-2.
- ^ Richard B. Wyrwas and Caroline Chick Jarrold (2006), "Production of C6O6− from Oligomerization of CO on Molybdenum Anions". Journal of the American Chemical Society, volume 128, issue 42, pages 13688–13689 PubMed doi:10.1021/ja0643927
- ^ Thomas M. Klapötke; Kurt Polborn and Jan J. Weigand (2005). "Dodecahydroxycyclohexane dihydrate". Acta Crystallographica E 61: o1393. doi:10.1107/S1600536805010007.
- ^ Willis B. Person and Dale G. Williams (1957), "Infrared Spectra and the Structure of Leuconic Acid and Triquinoyl". Journal of Physical Chemistry, volume 61, pages 1017-1018 doi:10.1021/j150553a047
- ^ Howard E Worne, U.S. Patent 3,227,641 "Polycarbonyls", issued Jan 4, 1966
- ^ William J. Hale (1949), "Farmer Victorious"
- ^ William W. Goodrich (1986), interview for FDA History (Part 2)
- ^ U. S. Food and Drug Administration (1989), Import Alert #66-46 – Unapproved Version of Rodaquin
- ^ Renewal & Wellness, LLC (2009) Protocel, an alternative medicine claimed to contain triquinoyl. Last accessed on March 25, 2009
See also
- Cyclopentanepentone
- Ethylenetetracarboxylic dianhydride, an isomer of C6O6.
Oxocarbons Common oxides Exotic oxides Compounds derived from oxides Categories:- Oxocarbons
Wikimedia Foundation. 2010.
