- Carbide
-
- For the software development tool targeting the Symbian OS, see Carbide.c++.
In chemistry, a carbide is a compound composed of carbon and a less electronegative element. Carbides can be generally classified by chemical bonding type as follows: (i) salt-like, (ii) covalent compounds, (iii) interstitial compounds, and (iv) "intermediate" transition metal carbides. Examples include calcium carbide, silicon carbide, tungsten carbide (often called simply carbide), and cementite,[1] each used in key industrial applications.
Contents
Salt-like carbides
Salt-like carbides are composed of highly electropositive elements such as the alkali metals, alkaline earths, and group 3 metals including scandium, yttrium and lanthanum. Aluminium from group 13 forms carbides, but gallium, indium and thallium do not. These materials feature isolated carbon centers, often described as "C4−", in the methanides or methides; two atom units, "C22−" in the acetylides; and three atom units "C34−" in the sesquicarbides.[1] The naming of ionic carbides is not consistent and can be quite confusing.
Methanides
Carbides of this class decompose in water producing methane. Two such examples are aluminium carbide Al4C3 and beryllium carbide Be2C.
Methanides in general chemical context refers to any compound that hydrolyzes to methane, which might include also salts with hydrogenated anions such as CH3−, CH2−
2, and CH−
3. However, according to IUPAC systematic naming conventions, only the last is properly called "methanide". In theory one can describe compounds that contain the methyl group, with relatively large bond polarity between the carbon and non-hydrogen atom, as salts of this anion; however in truth most such compounds, if not all, are, in fact covalent.Acetylides
Several carbides are assumed to be salts of the acetylide anion C22– (also called percarbide), which has a triple bond between the two carbon atoms. Alkali metals, alkaline earth metals, and lanthanoid metals form acetylides, e.g., sodium carbide Na2C2, calcium carbide CaC2, and LaC2.[1] Lanthanoids also form acetylides with formula M2C3. Metals from group 11 too tend to form acetylides, such as copper(I) acetylide and silver acetylide. Carbides of the actinide elements, which have stoichiometry MC2 and M2C3, are also described as salt-like derivatives of C22–.
The C-C triple bond length ranges from 109.2 pm in CaC2 (similar to ethyne), to 130.3 pm in LaC2 and 134 pm in UC2. The bonding in LaC2 has been described in terms of LaIII with the extra electron delocalised into the antibonding orbital on C22−, explaining the metallic conduction.[1]
Sesquicarbides
The polyatomic ion C34–, sometimes called sesquicarbide, is found in Li4C3, Mg2C3. The ion is linear and is isoelectronic with CO2.[1] The C-C distance in Mg2C3 is 133.2 pm.[2] Mg2C3 yields methylacetylene, CH3CCH, on hydrolysis which was the first indication that it may contain C34–.
Covalent carbides
The carbides of silicon and boron are described as "covalent carbides", although virtually all compounds of carbon exhibit some covalent character. Silicon carbide has two similar crystalline forms, which are both related to the diamond structure.[1] Boron carbide, B4C, on the other hand has an unusual structure which includes icosahedral boron units linked by carbon atoms. In this respect boron carbide is similar to the boron rich borides. Both silicon carbide, SiC, (carborundum) and boron carbide, B4C are very hard materials and refractory. Both materials are important industrially. Boron also forms other covalent carbides, e.g. B25C.
Interstitial carbides
The carbides of the group 4, 5 and 6 transition metals (with the exception of chromium) are often described as interstitial compounds.[1] These carbides have metallic properties and are refractory. Some exhibit a range of stoichiometries, e.g. titanium carbide, TiC. Titanium carbide and tungsten carbide are important industrially and are used to coat metals in cutting tools.[3]
The longheld view is that the carbon atoms fit into octahedral interstices in a close packed metal lattice when the metal atom radius is greater than approximately 135 pm:[1]
- When the metal atoms are cubic close packed, (ccp), then filling all of the octahedral interstices with carbon achieves 1:1 stoichiometry with the rock salt structure, (note that in rock salt, NaCl, it is the chloride anions that are cubic close packed).
- When the metal atoms are hexagonal close packed, (hcp), as the octahedral interstices lie directly opposite each other on either side of the layer of metal atoms, filling only one of these with carbon achieves 2:1 stoichiometry with the CdI2 structure.
The following table[1][3] shows actual structures of the metals and their carbides. (N.B. the body centred cubic structure adopted by vanadium, niobium, tantalum, chromium, molybdenum and tungsten is not a close packed lattice.) The notation "h/2" refers to the M2C type structure described above, which is only an approximate description of the actual structures. The simple view that the lattice of the pure metal "absorbs" carbon atoms can be seen to be untrue as the packing of the metal atom lattice in the carbides is different from the packing in the pure metal, although it is technically correct that the carbon atoms fit into the octahedral interstices of a close-packed metal lattice.
Metal Structure of pure metal Metallic
radius (pm)MC
metal atom packingMC structure M2C
metal atom packingM2C structure Other carbides titanium hcp 147 ccp rock salt zirconium hcp 160 ccp rock salt hafnium hcp 159 ccp rock salt vanadium cubic body centered 134 ccp rock salt hcp h/2 V4C3 niobium cubic body centered 146 ccp rock salt hcp h/2 Nb4C3 tantalum cubic body centered 146 ccp rock salt hcp h/2 Ta4C3 chromium cubic body centered 128 Cr23C6, Cr3C,
Cr7C3, Cr3C2molybdenum cubic body centered 139 hexagonal hcp h/2 Mo3C2 tungsten cubic body centered 139 hexagonal hcp h/2 For a long time the non-stoichiometric phases were believed to be disordered with a random filling of the interstices, however short and longer range ordering has been detected.[4]
Intermediate transition metal carbides
In these carbides, the transition metal ion is smaller than the critical 135 pm, and the structures are not interstitial but are more complex. Multiple stoichiometries are common, for example iron forms a number of carbides, Fe3C, Fe7C3 and Fe2C. The best known is cementite, Fe3C, which is present in steels. These carbides are more reactive than the interstitial carbides, for example the carbides of Cr, Mn, Fe, Co and Ni all are hydrolysed by dilute acids and sometimes by water, to give a mixture of hydrogen and hydrocarbons. These compounds share features with both the inert interstitials and the more reactive salt-like carbides.[1]
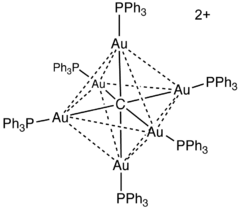
Molecular carbides
Metal complexes containing Cn fragments are well known. Most common are carbon-centered clusters, such as [Au6C(PPh3)6]2+. Similar species are known for the metal carbonyls and the early metal halides. Even terminal carbides have been crystallized, e.g., CRuCl2(P(C6H11)3)2.
Impossible carbides
Some metals, such as lead and tin, are believed not to form carbides under any circumstances.[5] There exists however a mixed titanium-tin carbide, which is a two-dimensional conductor.[6] (In 2007, there were two reports of a lead carbide PbC2, apparently of the acetylide type; but these claims have yet to be published in reviewed journals.)
Related materials
In addition to the carbides, other groups of related carbon compounds exist:[1]
- graphite intercalation compounds
- alkali metal fullerides
- endohedral fullerenes, where the metal atom is encapsulated inside a fullerene molecule
- metallacarbohedrenes(met-cars) which are cluster compounds containing C2 units.
- tunable nanoporous carbon, where gas chlorination of metallic carbides removes metal molecules to form a highly porous, near-pure carbon material capable of high-density energy storage.
- Transition metal carbene complexes.
References
- ^ a b c d e f g h i j k Greenwood, Norman N.; Earnshaw, A. (1984). Chemistry of the Elements. Oxford: Pergamon. pp. 318–22. ISBN 0-08-022057-6.
- ^ Fjellvag H. and Pavel K. (1992). "Crystal Structure of Magnesium Sesquicarbide". Inorg. Chem. 31 (15): 3260. doi:10.1021/ic00041a018.
- ^ a b Peter Ettmayer & Walter Lengauer (1994). "Carbides: transition metal solid state chemistry". In R. Bruce King. Encyclopedia of Inorganic Chemistry. John Wiley & Sons. ISBN 0-471-93620-0.
- ^ C.H. de Novion and J.P. Landesman (1985). "Order and disorder in transition metal carbides and nitrides: experimental and theoretical aspects" (free download pdf). Pure & Appl. Chem. 57 (10): 1391. doi:10.1351/pac198557101391.
- ^ John Percy (1870). The Metallurgy of Lead, including Desiverization and Cupellation. London: J. Murray. p. 67. http://www.archive.org/stream/metallurgyleadi01percgoog#page/n86/mode/2up/.
- ^ Y. C. Zhou, H. Y. Dong and B. H. Yu (2000). "Development of two-dimensional titanium tin carbide (Ti2SnC) plates based on the electronic structure investigation". Materials Research Innovations 4 (1): 36–41. doi:10.1007/s100190000065.
Inorganic compounds of carbon Oxides and related Other ionic compounds Carbides [:C≡C:]2– · Cyanides [:C≡N:]– · Cyanates [:O-C≡N:]– · Thiocyanates [:S-C≡N:]– · Fulminates [:C≡N-O:]– · Thiofulminates [:C≡N-S:]–Categories:- Carbides
- Anions
- Salts
Wikimedia Foundation. 2010.