- Copper(I) acetylide
-
Copper(I) acetylide 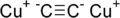 Dicuprous acetylide
Dicuprous acetylideIdentifiers CAS number 1117-94-8 PubChem 19021056 Jmol-3D images Image 1 - [C-]#[C-].[Cu+].[Cu+]
Properties Molecular formula C2Cu2 Molar mass 151.11 g/mol Hazards Main hazards Explosive, Harmful  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Copper(I) acetylide, or cuprous acetylide, is an inorganic chemical compound with the formula Cu2C2. It is a heat and shock sensitive high explosive, more sensitive than silver acetylide[citation needed]. It is a metal acetylide. It is similar to silver acetylide and calcium carbide, though it is not called carbide in literature. Though not practically useful due to high sensitivity and reactivity towards water[citation needed], it is interesting as a curiosity because it is one of the very few explosives that do not liberate any gaseous products upon detonation.
Copper acetylide can be prepared by passing acetylene gas through copper(I) chloride solution in presence of ammonia:
- C2H2 + 2CuCl → Cu2C2 + 2HCl
The reaction product is a reddish precipitate. The reaction is used as a test for acetylene.
Copper acetylide can form inside pipes made of copper or an alloy with high copper content, which may result in violent explosion.[1] This was found to be the cause of explosions in acetylene plants, and led to abandonment of copper as a construction material in such plants.[2] Copper catalysts used in petrochemistry can also possess a degree of risk under certain conditions.[3]
References
- ^ "Mine Safety and Health Administration (MSHA) - Accident Prevention Program - Miner's Tips - Hazards of Acetylene Gas". http://www.msha.gov/Accident_Prevention/Tips/acetylenegas.htm. Retrieved 2008-06-08.
- ^ [1][dead link]
- ^ "The Safe Use of Copper -Containing Catalysts in Ethylene Plants". http://aiche.confex.com/aiche/s06/preliminaryprogram/abstract_43768.htm. Retrieved 2008-06-08.

This explosives-related article is a stub. You can help Wikipedia by expanding it.
