- Copper(I) chloride
-
Copper(I) chloride 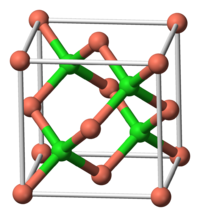 Copper(I) chlorideOther namesCuprous chloride
Copper(I) chlorideOther namesCuprous chlorideIdentifiers CAS number 7758-89-6 
PubChem 62652 ChemSpider 56403 
EC number 231-842-9 ChEBI CHEBI:53472 
RTECS number GL6990000 Jmol-3D images Image 1 - Cl[Cu]
Properties Molecular formula CuCl Molar mass 98.999 g/mol Appearance white powder, slightly green from oxidized impurities Density 4.145 g/cm3 Melting point 426 °C (703 K)
Boiling point 1490 °C (1760 K) (decomp.)
Solubility in water 0.0062 g/100 mL (20 °C) Solubility product, Ksp 1.72 x 10-7 Solubility insoluble in ethanol
acetone; soluble in concentrated HCl, NH4OHRefractive index (nD) 1.930[1] Structure Crystal structure Zinc blende structure Hazards MSDS JT Baker EU Index 029-001-00-4 EU classification Harmful (Xn)
Dangerous for the environment (N)R-phrases R22, R50/53 S-phrases (S2), S22, S60, S61 NFPA 704 Flash point Non-flammable LD50 140 mg/kg Related compounds Other anions Copper(I) bromide
Copper(I) iodideOther cations Copper(II) chloride
Silver(I) chloride chloride (verify) (what is:
chloride (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Copper(I) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula CuCl. The substance is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. Impure samples appear green due to the presence of copper(II) chloride.[2]
Contents
History
Copper(I) chloride was first prepared by Robert Boyle in the mid-seventeenth century[3] from mercury(II) chloride ("Venetian sublimate") and copper metal:
- HgCl2 + 2 Cu → 2 CuCl + Hg
In 1799, J.L. Proust characterized the two different chlorides of copper. He prepared CuCl by heating CuCl2 at red heat in the absence of air, causing it to lose half of its combined chlorine followed by removing residual CuCl2 by washing with water.[4]
An acidic solution of CuCl was formerly used for analysis of carbon monoxide content in gases, for example in Hempel's gas apparatus.[5] This application was significant[6] during the time that coal gas was widely used for heating and lighting, during the nineteenth and early twentieth centuries.
Chemical properties
Copper(I) chloride is a Lewis acid, which is classified as soft according to the Hard-Soft Acid-Base concept. Thus, it tends to form stable complexes with soft Lewis bases such as triphenylphosphine:
- CuCl + P(C6H5)3 → [CuCl(P(C6H5)3)]4
Although CuCl is insoluble in water, it dissolves in aqueous solutions containing suitable donor molecules. It forms complexes with halide ions, for example forming H3O+ CuCl2- with concentrated hydrochloric acid. It is attacked by CN-, S2O32-, and NH3 to give the corresponding complexes.
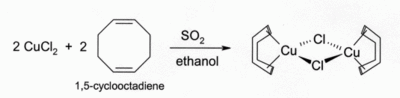
Solutions of CuCl in HCl or NH3 absorb carbon monoxide to form colourless complexes such as the chloride-bridged dimer [CuCl(CO)]2. The same hydrochloric acid solutions also react with acetylene gas to form [CuCl(C2H2)]. Ammoniacal solutions of CuCl react with acetylenes to form the explosive copper(I) acetylide. Complexes of CuCl with alkenes can be prepared by reduction of CuCl2 by sulfur dioxide in the presence of the alkene in alcohol solution. Complexes with dienes such as 1,5-cyclooctadiene are particularly stable:[7]
In absence of other ligands, its aqueous solutions are unstable with respect to disproportionation into Cu and CuCl2.[8] In part for this reason samples in air assume a green coloration (see photograph in upper right).
Uses
The main use of copper(I) chloride is as a precursor to the fungicide copper oxychloride. For this purpose aqueous copper(I) chloride is generated by comproportionation and then air-oxidized:
- Cu + CuCl2 → 2 CuCl
- 6 CuCl + 3/2 O2 + 3 H2O → 2 Cu3Cl2(OH)4 + CuCl2
Copper(I) chloride catalyzes a variety of organic reactions, as discussed above. Its affinity for carbon monoxide in the presence of aluminium chloride is exploited in the COPureSM process.
In organic synthesis
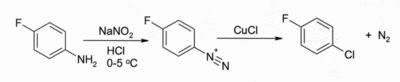
In the Sandmeyer reaction.[9] Treatment of an arenediazonium salt with CuCl leads to an aryl chloride, for example:
The reaction has wide scope and usually gives good yields.
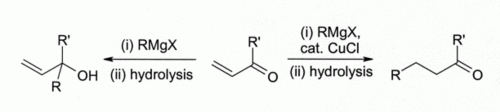
Early investigators observed that copper(I) halides catalyse 1,4-addition of Grignard reagents to alpha,beta-unsaturated ketones[10] led to the development of organocuprate reagents that are widely used today in organic synthesis:[11]
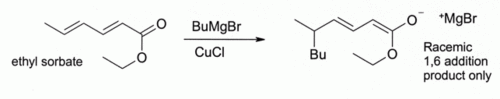
This finding led to the development of organocopper chemistry. For example, CuCl reacts with methyllithium (CH3Li) to form "Gilman reagents" such as (CH3)2CuLi, which find extensive use in organic synthesis. Grignard reagents form similar organocopper compounds. Although other copper(I) compounds such as copper(I) iodide are now more often used for these types of reactions, copper(I) chloride is still recommended in some cases:[12]
Here, Bu indicates an n-butyl group. Without CuCl, the Grignard reagent alone gives a mixture of 1,2- and 1,4-addition products (i.e., the butyl adds at the C closer to the C=O).
Copper(I) chloride is also an intermediate formed from copper(II) chloride in the Wacker process.
In polymer chemistry
CuCl is used as a catalyst in Atom Transfer Radical Polymerization (ATRP).
References
- ^ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0-07-049439-8
- ^ United States Patent US4582579 "method of preparing cupric ion free cuprous chloride" Section 2, lines 4-41 , via www.freepatentsonline.com
- ^ Boyle, Robert (1666). Considerations and experiments about the origin of forms and qualities. Oxford. As reported in Mellor.
- ^ Proust, J. L. (1799). Ann. Chim. Phys. (1) 32: 26.
- ^ Martin, Geoffrey (1917). Industrial and Manufacturing Chemistry (Part 1, Organic ed.). London: Crosby Lockwood. pp. 330–31.
- ^ Lewes, Vivian H. (1891). "Journal of the Society of Chemical Industry". Journal of the Society of Chemical Industry 10: 407–413. http://books.google.com/?id=oSEAAAAAMAAJ&pg=PA408&lpg=PA407#v=onepage&q.
- ^ Nicholls, D. Complexes and First-Row Transition Elements, Macmillan Press, London, 1973.
- ^ Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements, 2nd ed., Butterworth-Heinemann, Oxford, UK, 1997.
- ^ (a) Wade, L. G. Organic Chemistry, 5th ed., p. 871, Prentice Hall, Upper Saddle RIver, New Jersey, 2003. (b) March, J. Advanced Organic Chemistry, 4th ed., p. 723, Wiley, New York, 1992.
- ^ Kharasch, M. S; Tawney, P. O (1941). "Factors Determining the Course and Mechanisms of Grignard Reactions. II. The Effect of Metallic Compounds on the Reaction between Isophorone and Methylmagnesium Bromide". J. Am. Chem. Soc. 63 (9): 2308. doi:10.1021/ja01854a005.
- ^ Jasrzebski, J. T. B. H.; van Koten, G. in Modern Organocopper Chemistry, (N. Krause, ed.), p. 1, Wiley-VCH, Weinheim, Germany, 2002.
- ^ (a) Bertz, S. H.; Fairchild, E. H. in Handbook of Reagents for Organic Synthesis, Volume 1: Reagents, Auxiliaries and Catalysts for C-C Bond Formation, (R. M. Coates, S. E. Denmark, eds.), pp. 220-3, Wiley, New York, 1999. (b) Munch-Petersen, J., et al., Acta Chimica Scand., 15, 277 (1961).
Further reading
- Mellor, J. W., A Comprehensive Treatise on Inorganic and Theoretical Chemistry, Volume III, pp157-168. Longmans, Green & Co., London, 1967 (new impression).
External links
- National Pollutant Inventory - Copper and compounds fact sheet
- The COPureSM Process for purifying CO utilizing a copper chloride complex
Copper compounds Categories:- Chlorides
- Copper compounds
- Metal halides
- Coordination compounds
- Pyrotechnic colorants
Wikimedia Foundation. 2010.