- Copper(II) oxide
-
Copper(II) oxide  Copper(II) oxideOther namesCupric oxide
Copper(II) oxideOther namesCupric oxideIdentifiers CAS number 1317-38-0 
PubChem 164827 ChemSpider 144499 
UNII V1XJQ704R4 
RTECS number GL7900000 Jmol-3D images Image 1
Image 2- [Cu]=O
[Cu+2].[O-2]
Properties Molecular formula CuO Molar mass 79.545 g/mol Appearance black powder Density 6.31 g/cm3 Melting point 1201 °C (1474 K)
Boiling point 2000 °C
Solubility in water insoluble Solubility in Ammonium hydroxide soluble Band gap 1.2 eV Structure Crystal structure monoclinic, mS8[1] Space group C2/c, #15 Lattice constant a = 4.6837, b = 3.4226, c = 5.1288 Lattice constant α = 90°, β = 99.54°, γ = 90° Hazards MSDS Fischer Scientific EU Index Not listed EU classification Harmful (Xn)
Dangerous for the environment (N)NFPA 704 Flash point Non-flammable Related compounds Other anions Copper(II) sulfide Other cations Nickel(II) oxide
Zinc oxideRelated compounds Copper(I) oxide  oxide (verify) (what is:
oxide (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Copper(II) oxide or cupric oxide (CuO) is the higher oxide of copper. As a mineral, it is known as tenorite.
Contents
Chemistry
It is a black solid with an ionic structure which melts above 1200 °C with some loss of oxygen. It can be formed by heating copper in air:
- 2 Cu + O2 → 2 CuO
Here, it is formed along with copper(I) oxide as a side product; thus, it is better prepared by heating copper(II) nitrate, copper(II) hydroxide or copper(II) carbonate:
- 2 Cu(NO3)2 → 2 CuO + 4 NO2 + O2
- Cu(OH)2 (s) → CuO (s) + H2O (l)
- CuCO3 → CuO + CO2
Copper(II) oxide is a basic oxide, so it dissolves in mineral acids such as hydrochloric acid, sulfuric acid or nitric acid to give the corresponding copper(II) salts:
- CuO + 2 HNO3 → Cu(NO3)2 + H2O
- CuO + 2 HCl → CuCl2 + H2O
- CuO + H2SO4 → CuSO4 + H2O
It reacts with concentrated alkali to form the corresponding cuprate salts:
- 2 XOH + CuO + H2O → X2[Cu(OH)4]
It can also be reduced to copper metal using hydrogen or carbon monoxide:
- CuO + H2 → Cu + H2O
- CuO + CO → Cu + CO2
A laboratory method for preparing copper(II) oxide is to electrolyze water containing sodium bicarbonate at a moderate voltage with a copper anode, collect the mixture of copper hydroxide, basic copper carbonate, and copper carbonate produced, and heat it.
Crystal structure
Copper(II) oxide belongs to the monoclinic crystal system, with a crystallographic point group of 2/m or C2h. The space group of its unit cell is C2/c, and its lattice parameters are a = 4.6837(5), b = 3.4226(5), c = 5.1288(6), α = 90° , β = 99.54(1)°, γ = 90°.[1] The copper atom is coordinated by 4 oxygen atoms in an approximately square planar configuration.[1]
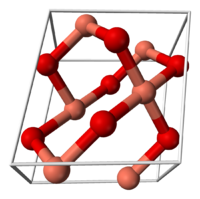
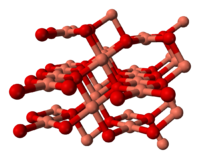
the unit cell of copper(II) oxide part of the crystal structure of CuO Health effects
Copper(II) oxide is an irritant. It also can cause damage to the endocrine and central nervous system. Contact to the eyes or skin can cause irritation. Ingesting cupric oxide powder can result in a metallic taste, nausea, vomiting and stomach pain. In more severe cases, there may be blood in vomit or black or tarry stools, jaundice and enlarged liver. Blood cells rupture resulting in circulatory collapse and shock. Inhalation can lead to damage to the lungs and septum. Inhalation of fumes during smelting of cupric oxide powder can lead to a disease called metal fume fever, which can result in flu like symptoms. Copper (II) oxide can cause a toxic build-up of copper in a small subset of the population with Wilson's disease. Handling copper (II) oxide powder should be done in well ventilated area, and care should be taken to avoid contact with the skin or eyes.[2] However copper is an essential trace element for the normal function of many tissues, including the nervous system, immune system, heart, skin and for the formation of capillaries [3][4] as well as copper being extremely well metabolized by humans. Copper oxide is used in vitamins supplements as a safe source of copper[5] and over-the-counter treatments. Copper oxide is also used in consumer products such as pillowcases and socks, due to its cosmetic and anti-microbial properties.[6][7][8][9] The risk of dermal sensitivity to copper is considered extremely minimal.[10]
Uses
Cupric oxide is used as a pigment in ceramics to produce blue, red, and green (and sometimes gray, pink, or black) glazes. It is also used to produce cuprammonium hydroxide solutions, used to make rayon. It is also occasionally used as a dietary supplement in animals, against copper deficiency.[11] Copper(II) oxide has application as a p-type semiconductor, because it has a narrow band gap of 1.2 eV. It is an abrasive used to polish optical equipment. Cupric oxide can be used to produce dry cell batteries. It has also been used in wet cell batteries as the cathode, with lithium as an anode, and dioxalane mixed with lithium perchlorate as the electrolyte. Copper(II) oxide can be used to produce other copper salts. It is also used when welding with copper alloys.[12]
Another use for cupric oxide is as a substitute for iron oxide in thermite. This can turn the thermite from an incendiary to a low explosive.
Use in disposal
Cupric oxide can be used to safely dispose of hazardous materials such as cyanide, hydrocarbons, halogenated hydrocarbons and dioxins, through oxidation.[13]
Here are equations depicting the decomposition of phenol and pentachlorophenol, respectively, with copper oxide:
- C6H5OH + 14CuO → 6CO2 + 3H2O + 14Cu
- C6Cl5OH + 2H2O + 9CuO → 6CO2 + 5HCl + 9Cu
Properties
- Work function:5.3eV.[14]
See also
References
- ^ a b c The effect of hydrostatic pressure on the ambient temperature structure of CuO, Forsyth J.B., Hull S., J. Phys.: Condens. Matter 3 (1991) 5257-5261 , doi:10.1088/0953-8984/3/28/001
- ^ "Material safety data sheet: Copper (II) oxide". Iowa State University. 2003. http://avogadro.chem.iastate.edu/MSDS/CuO.html. Retrieved 2007-01-26.
- ^ Uauy et al. (1998). "Essentiality of copper in humans". Am J Clin Nutr 67 (3): 952S–9S. PMID 9587135. http://www.ajcn.org/cgi/content/abstract/67/5/952S.
- ^ Sen et al. (2002). "Copper-induced vascular endothelial growth factor expression and wound healing". Am J Physiol Heart Circ Physiol 282 (5): H1821–27. doi:10.1152/ajpheart.01015.2001. PMID 11959648. http://ajpheart.physiology.org/cgi/content/abstract/282/5/H1821.
- ^ "Vitamin Supplements:". http://www.empr.com/vitaminsmineralssupplements/centrum/drug/5901.
- ^ Borkow G et al. (2009). "Improvement of facial skin characteristics using copper oxide containing pillowcases: a double-blind, placebo-controlled, parallel, randomized study". Int J Cosmet Sci 31 (6): 437–43. doi:10.1111/j.1468-2494.2009.00515.x. PMID 19467028.
- ^ Borkow G et al. (2009). "Reducing the risk of skin pathologies in diabetics by using copper impregnated socks". Med Hypotheses 73 (6): 883–6. doi:10.1016/j.mehy.2009.02.050. PMID 19559540.
- ^ Borkow G et al. (2005). "Copper as a biocidal tool". Curr Med Chem 12 (18): 2163–75. doi:10.2174/0929867054637617. PMID 16101497.
- ^ Borkow G et al. (2004). "Copper-impregnated products with potent biocidal activities". FASEB J. 18 (14): 1728–30. doi:10.1096/fj.04-2029fje. PMID 15345689.
- ^ Hostynek JJ, and Maibach HI (2003). "hypersensitivity: dermatologic aspects-an overview". Rev Environ Health 153 (3): 153–83. PMID 14672513.
- ^ "Uses of Copper Compounds: Other Copper Compounds". Copper Development Association. 2007. http://www.copper.org/applications/compounds/other_compounds.html. Retrieved 2007-01-27.
- ^ "Cupric Oxide Data Sheet". Hummel Croton Inc.. 2006-04-21. http://www.axxousa.com/data/cuox_d.html. Retrieved 2007-02-01.
- ^ Kenney, Charlie W.; Uchida, Laura A. (April). "Use of copper (II) oxide as source of oxygen for oxidation reactions". http://www.freepatentsonline.com/4582613.html. Retrieved 2007-06-29
- ^ F. P. Koffyberg and F. A. Benko (1982). "A photoelectrochemical determination of the position of the conduction and valence band edges of p-type CuO". J. Appl. Phys. 53 (2): 1173. doi:10.1063/1.330567.
External links
Copper compounds Categories:- Oxides
- Copper compounds
- Semiconductor materials
- Pyrotechnic colorants
- [Cu]=O
Wikimedia Foundation. 2010.

