- Copper monosulfide
-
Copper monosulfide Copper sulfideOther namesIdentifiers CAS number 1317-40-4 
PubChem 14831 ChemSpider 14145 
RTECS number GL8912000 Jmol-3D images Image 1 - [Cu]=S
Properties Molecular formula CuS Molar mass 95.611 g/mol Density 4.6 g/cm3 Melting point above 500°C (decomposes [2])
Solubility in water insoluble Solubility product, Ksp 1.27 x 10-36 Solubility soluble in HNO3, NH4OH, KCN
soluble in HCl, H2SO4Refractive index (nD) 1.45 [1] Related compounds Other anions Copper(II) oxide Other cations zinc sulfide  monosulfide (verify) (what is:
monosulfide (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Copper monosulfide is a chemical compound of copper and sulfur. It occurs in nature as the dark indigo blue mineral covellite. It is a moderate conductor of electricity. [3] A black colloidal precipitate of CuS is formed when hydrogen sulfide, H2S, is bubbled through solutions of Cu(II) salts.[4] It is one of a number of binary compounds of copper and sulfur (see copper sulfide for an overview of this subject), and has attracted interest because of its potential uses in catalysis[5] and photovoltaics.[6]
CuS structure and bonding
Copper monosulfide crystallizes in the hexagonal crystal system, and this is the form of the mineral covellite. There is also an amorphous high pressure form [7] which on the basis of the Raman spectrum has been described as having a distorted covellite structure. An amorphous room temperature semiconducting form produced by the reaction of a Cu(II) ethylenediamine complex with thiourea has been reported, which transforms to the crystalline covellite form at 30 °C.[8]
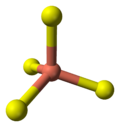
The crystal structure of covellite has been reported several times,[9][10][11] and whilst these studies are in general agreement on assigning the space group P63/mmc there are small discrepancies in bond lengths and angles between them. The structure was described as "extraordinary" by Wells[12] and is quite different from copper(II) oxide, but similar to CuSe (klockmannite). The covellite unit cell contains 6 formula units (12 atoms)in which:- 4 Cu atoms have tetrahedral coordination (see illustration).
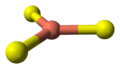
- 2 Cu atoms have trigonal planar coordination (see illustration).
- 2 pairs of S atoms are only 207.1 pm apart [11] indicating the existence of an S-S bond (a disulfide unit).
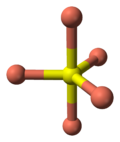
- the 2 remaining S atoms form trigonal planar triangles around the copper atoms, and are surrounded by five Cu atoms in a pentagonal bipyramid (see illustration).
- The S atoms at each end of a disulfide unit are tetrahedrally coordinated to 3 tetrahedrally coordinated Cu atoms and the other S atom in the disulfide unit (see illustration).
The formulation of copper monosulfide as CuIIS (i.e. containing no sulfur-sulfur bond) is clearly incompatible with the crystal structure, and also at variance with the observed diamagnetism [13] as a Cu(II) compound would have a d9 configuration and be expected to be paramagnetic.[4]
Studies using XPS[14][15][16][17] indicate that all of the copper atoms have an oxidation state of +1. This contradicts a formulation based on the crystal structure and obeying the octet rule that is found in many textbooks (e.g. [4][18]) describing CuS as containing both CuI and CuII i.e. (Cu+)2Cu2+(S2)2–S2–. An alternative formulation as (Cu+)3(S2–)(S2)– was proposed and supported by calculations.[19] The formulation should not be interpreted as containing radical anion, but rather that there is a delocalized valence "hole". [19][20] Electron paramagnetic resonance studies on the precipitation of Cu(II) salts indicates that the reduction of Cu(II) to Cu(I) occurs in solution. [21]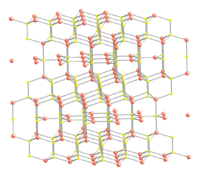
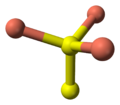
ball-and-stick model of part of
the crystal structure of covellitetrigonal planar
coordination of coppertetrahedral
coordination of coppertrigonal bipyramidal
coordination of sulfurtetrahedral
coordination of sulfur-note disulfide unitSee also
- Copper sulfide for an overview of all copper sulfide phases
- copper(I) sulfide, Cu2S
- Covellite
References
- ^ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0070494398
- ^ Blachnik, R.; Müller, A. (2000). "The formation of Cu2S from the elements I. Copper used in form of powders". Thermochimica Acta 361 (1–2): 31–52. doi:10.1016/S0040-6031(00)00545-1
- ^ Wells A.F. (1962) Structural Inorganic Chemistry 3d edition Oxford University Press
- ^ a b c Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. ISBN 0080379419.
- ^ Kuchmii, S.Y.; Korzhak A.V., Raevskaya A.E.,Kryukov A.I. (2001). "Catalysis of the Sodium Sulfide Reduction of Methylviologene by CuS Nanoparticles". Theoretical and Experimental Chemistry (New York: Springer) 37 (1): 36–41. doi:10.1023/A:1010465823376.
- ^ Mane, R.S.; Lokhande C.D. (June 2000). "Chemical deposition method for metal chalcogenide thin films". Materials Chemistry and Physics 65 (1): 1–31. doi:10.1016/S0254-0584(00)00217-0.
- ^ Peiris, M; Sweeney, J.S.; Campbell, A.J.; Heinz D. L. (1996). "Pressure-induced amorphization of covellite, CuS". J. Chem. Phys. 104 (1): 11–16. doi:10.1063/1.470870.
- ^ Grijalva, H.; Inoue, M.; Boggavarapu, S.; Calvert, P. (1996). "Amorphous and crystalline copper sulfides, CuS". J. Mater. Chem. 6 (7): 1157–1160. doi:10.1039/JM9960601157.
- ^ Oftedal, I. (1932). Z. Kristallogr. 83: 9–25.
- ^ Berry, L. G. (1954). "The crystal structure of covellite CuS and klockmannite CuSe". American Mineralogist 39: 504.
- ^ a b Evans, H.T. Jr.; Konnert J. (1976). "Crystal structure refinement of covellite". American Mineralogist 61: 996–1000.
- ^ Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
- ^ Magnetic susceptibility of the elements and inorganic compounds
- ^ Nakai, I.; Sugitani, Y.; Nagashima, K.; Niwa, Y. (1978). "X-ray photoelectron spectroscopic study of copper minerals". Journal of Inorganic and Nuclear Chemistry 40 (5): 789–791. doi:10.1016/0022-1902(78)80152-3.
- ^ Folmer, J.C.W.; Jellinek F. (1980). "The valence of copper in sulfides and selenides: An X-ray photoelectron spectroscopy study". Journal of the Less Common Metals 76 (1-2): 789–791. doi:10.1016/0022-5088(80)90019-3.
- ^ Folmer, J.C.W.; Jellinek F., Calis G.H.M (1988). "The electronic structure of pyrites, particularly CuS2 and Fe1−xCuxSe2: An XPS and Mössbauer study". Journal of Solid State Chemistry 72 (1): 137–144. doi:10.1016/0022-4596(88)90017-5.
- ^ Goh, S.W.; Buckley A.N., Lamb R.N. (February 2006). "Copper(II) sulfide?". Minerals Engineering 19 (2): 204–208. doi:10.1016/j.mineng.2005.09.003.
- ^ Cotton, F. Albert; Wilkinson, Geoffrey; Murillo, Carlos A.; Bochmann, Manfred (1999), Advanced Inorganic Chemistry (6th ed.), New York: Wiley-Interscience, ISBN 0-471-19957-5
- ^ a b Liang, W.; Whangbo M, -H (February 1993). "Conductivity anisotropy and structural phase transition in Covellite CuS". Solid State Communications 85 (5): 405–408. doi:10.1016/0038-1098(93)90689-K.
- ^ Nozaki, H; Shibata, K; Ohhashi,N. (April 1991). "Metallic hole conduction in CuS". Journal of Solid State Chemistry 91 (2): 306–311. doi:10.1016/0022-4596(91)90085-V.
- ^ Luther, GW; Theberge SM, Rozan TF, Rickard D, Rowlands CC, Oldroyd A. (February 2002). "Aqueous copper sulfide clusters as intermediates during copper sulfide formation.". Environ. Sci. Technol. 36 (3): 394–402. doi:10.1021/es010906k. PMID 11871554.
Copper compounds Categories:- Sulfides
- Copper compounds
- Mixed valence compounds
Wikimedia Foundation. 2010.

