- Polyatomic ion
-
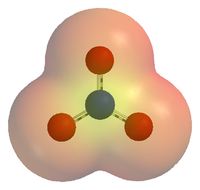 An electrostatic potential map of the nitrate ion (NO3−). Areas coloured red are lower in energy than areas coloured yellow
An electrostatic potential map of the nitrate ion (NO3−). Areas coloured red are lower in energy than areas coloured yellow
A polyatomic ion, also known as a molecular ion, is a charged species (ion) composed of two or more atoms covalently bonded or of a metal complex that can be considered as acting as a single unit in the context of acid and base chemistry or in the formation of salts. The prefix "poly-" means "many," in Greek, but even ions of two atoms are commonly referred to as polyatomic. In older literature, a polyatomic ion is also referred to as a radical, and less commonly, as a radical group. In contemporary usage, the term radical refers to free radicals which are (not necessarily charged) species with an unpaired electron.
For example, a hydroxide ion is made of one oxygen atom and one hydrogen atom: its chemical formula is (OH)−. It has a charge of −1. An ammonium ion is made up of one nitrogen atom and four hydrogen atoms: its chemical formula is (NH4)+. It has charge of +1.
A polyatomic ion can often be considered as the conjugate acid or conjugate base of a neutral molecule. For example the sulfate anion, SO42−, is derived from H2SO4 which can be regarded as SO3 + H2O.
Contents
Nomenclature
There are two "rules" that can be used for learning the nomenclature of polyatomic ions. First, when the prefix bi- is added to a name, a hydrogen is added to the ion's formula and its charge is increased by 1, the latter being a consequence of the hydrogen ion carrying a +1 charge. An alternate to the bi- prefix is to use the word hydrogen in its place: the anion derived from H+ + CO32−, HCO3− can be called either bicarbonate or hydrogen carbonate.
Note that many of the common polyatomic anions are conjugate bases of acids derived from the oxides of non-metallic elements. For example the sulfate anion, SO42−, is derived from H2SO4 which can be regarded as SO3 + H2O.
The second rule looks at the number of oxygens in an ion. Consider the chlorine oxoanion family:
oxidation state −1 +1 +3 +5 +7 anion name chloride hypochlorite chlorite chlorate perchlorate formula Cl− ClO− ClO2− ClO3− ClO4− structure 
First, think of the -ate ion as being the "base" name, in which case the addition of a per- prefix adds an oxygen. Changing the -ate suffix to -ite will reduce the oxygens by one, and keeping the suffix -ite and adding the prefix hypo- reduces the number of oxygens by two. In all situations, the charge is not affected. The naming pattern follows within many different oxyanion series based on a standard root for that particular series. The -ite has one less oxygen than the -ate, but different -ate anions might have different numbers of oxygen atoms.
These rules will not work with all polyatomic ions, but they do work with the most common ones (sulfate, phosphate, nitrate, chlorate).
Examples of common polyatomic ions
The following tables give examples of commonly-encountered polyatomic ions. Only a few representatives are given, as the number of polyatomic ions encountered in practice is very large.
Anions Acetate (ethanoate) CH3COO− or C2H3O−
2Benzoate C6H5COO− or C7H5O−
2Bicarbonate (hydrogen carbonate) HCO−
3Carbonate CO2−
3Cyanide CN− Hydroxide OH− Nitrate NO−
3Phosphate PO3−
4Sulfate SO2−
4Cations Ammonium NH+
4Hydronium H3O+ Mercury(I) Hg2+
2Tropylium C7H+
7See also
External links
Categories:- Ions
Wikimedia Foundation. 2010.
