- Caesium oxide
-
Caesium oxide[1][2] 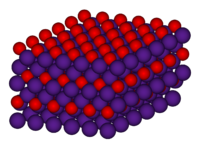 Caesium oxideOther namesCesium oxide (US)
Caesium oxideOther namesCesium oxide (US)Identifiers CAS number 20281-00-9 
PubChem 9903865 ChemSpider 8079519 
EC-number 243-679-0 Jmol-3D images Image 1 - [Cs+].[Cs+].[O-2]
Properties Molecular formula Cs2O Molar mass 281.81 g/mol Appearance yellow-orange solid Density 4.65 g/cm3, solid Melting point 490 °C (under N2)
Solubility in water reacts Structure Crystal structure anti-CdCl2 (hexagonal) Thermochemistry Std enthalpy of
formation ΔfHo298-345.8 kJ/mol Standard molar
entropy So298146.9 J K-1 mol-1 Specific heat capacity, C 76.0 J K-1 mol-1 Hazards EU Index not listed Flash point non-flammable Related compounds Other anions Caesium hydroxide Other cations Lithium oxide
Sodium oxide
Potassium oxide
Rubidium oxide oxide (verify) (what is:
oxide (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Caesium oxide (IUPAC name) or cesium oxide describes inorganic compounds composed of caesium and oxygen. The following binary (containing only Cs and O) oxides of caesium are known: Cs11O3, Cs4O, Cs7O, and Cs2O.[3] Both the oxide and suboxides are brightly coloured. The species Cs2O forms yellow-orange hexagonal crystals.[1]
Uses
Caesium oxide is used in photocathodes to detect infrared signals in devices such as image intensifiers, vacuum photodiodes, photomultipliers, and TV camera tubes[4] L. R. Koller described the first modern photoemissive surface in 1929–30 as a layer of caesium on a layer of caesium oxide on a layer of silver.[5] It is a good electron emitter; however, its high vapor pressure limits its usefulness.[6]
Reactions
Elemental magnesium reduces caesium oxide to elemental caesium, forming magnesium oxide as a side-product:[7][8]
- Cs2O + Mg → Cs + MgO
Cs2O is hygroscopic, forming the corrosive CsOH on contact with water.
References
- ^ a b Lide, David R., ed (2006). CRC Handbook of Chemistry and Physics (87th ed.). Boca Raton, FL: CRC Press. pp. 451, 514. ISBN 0-8493-0487-3..
- ^ Greenwood, Norman N.; Earnshaw, A. (1984). Chemistry of the Elements. Oxford: Pergamon. pp. 97–100. ISBN 0-08-022057-6..
- ^ Simon, A. (1997), "Group 1 and 2 Suboxides and Subnitrides — Metals with Atomic Size Holes and Tunnels", Coord. Chem. Rev. 163: 253–270, doi:10.1016/S0010-8545(97)00013-1.
- ^ Capper, Peter; Elliott, C. T. (2000), Infrared Detectors and Emitters, Springer, p. 14, ISBN 9780792372066, http://books.google.com/?id=HtgEcjQcgkkC&pg=PA14&dq=%22cesium+oxide%22+OR+%22caesium+oxide%22
- ^ Busch, Kenneth W.; Busch, Marianna A. (1990), Multielement Detection Systems for Spectrochemical Analysis, Wiley-Interscience, p. 12, ISBN 9780471819745, http://books.google.com/?id=9H0W1J-Rku4C&pg=PA12&dq=%22cesium+oxide%22+OR+%22caesium+oxide%22
- ^ Boolchand, Punit, ed. (2000), Insulating and Semiconducting Glasses, World Scientific, p. 855, ISBN 9789810236731, http://books.google.com/?id=QK2f4eVh7qgC&pg=PA855&dq=%22cesium+oxide%22+OR+%22caesium+oxide%22
- ^ Turner, Jr., Francis M., ed. (1920), The Condensed Chemical Dictionary, New York: Chemical Catalog Co., p. 121, http://books.google.com/?id=y8y0XE0nsYEC&pg=PA121&dq=%22cesium+oxide%22+OR+%22caesium+oxide%22
- ^ Arora, M.G. (1997), S-Block Elements, New Delhi: Anmol Publications, p. 13, ISBN 9788174885623, http://books.google.com/?id=QR3TCaKaykEC&pg=PA256&dq=%22Bromine+dioxide%22
Caesium compounds Categories:- Caesium compounds
- Oxides
- Inorganic compound stubs
Wikimedia Foundation. 2010.
