- Lithium oxide
-
Lithium oxide 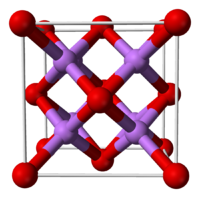 Lithium oxideOther namesLithium monoxide
Lithium oxideOther namesLithium monoxide
LithiaIdentifiers CAS number 12057-24-8 
PubChem 166630 ChemSpider 145811 
RTECS number OJ6360000 Jmol-3D images Image 1 - [Li+].[Li+].[O-2]
Properties Molecular formula Li2O Molar mass 29.88 g/mol Appearance white solid Density 2.013 g/cm3 Melting point 1570 °C, 1843 K, 2858 °F
Solubility in water decomposes
6.67 g/100 mL (0 °C)
10.02 g/100 mL (100 °C)log P 9.23 Refractive index (nD) 1.644 [1] Structure Crystal structure Antifluorite (cubic), cF12 Space group Fm3m, No. 225 Coordination
geometryTetrahedral (Li+); cubic (O2–) Thermochemistry Std enthalpy of
formation ΔfHo298-20.01 kJ/g Specific heat capacity, C 1.8105 J/g K Hazards EU Index Not listed Main hazards Corrosive, reacts violently with water Flash point Non-flammable Related compounds Other anions Lithium sulfide Other cations Sodium oxide
Potassium oxide
Rubidium oxide
Caesium oxideRelated lithium oxides Lithium peroxide
Lithium superoxideRelated compounds Lithium hydroxide  oxide (verify) (what is:
oxide (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Lithium oxide (Li2O) or lithia is an inorganic chemical compound. Lithium oxide is formed along with small amounts of lithium peroxide when lithium metal is burned in the air and combines with oxygen[2]:
- 4Li+O2 → 2Li2O.
Pure Li2O can be produced by the thermal decomposition of lithium peroxide, Li2O2 at 450°C[2]
- 2Li2O2 → 2Li2O + O2
Contents
Structure
In the solid state lithium oxide adopts an antifluorite structure which is related to the CaF2, fluorite structure with Li cations substituted for fluoride anions and oxide anions substituted for calcium cations.[3] The ground state gas phase Li2O molecule is linear with a bond length consistent with strong ionic bonding.[4][5] VSEPR theory would predict a bent shape similar to H2O.
Uses
Lithium oxide is used as a flux in ceramic glazes; and creates blues with copper and pinks with cobalt. Lithium oxide reacts with water and steam, forming lithium hydroxide and should be isolated from them.
Its usage is also being investigated for non-destructive emission spectroscopy evaluation and degradation monitoring within thermal barrier coating systems. It can be added as a co-dopant with yttria in the zirconia ceramic top coat, without a large decrease in expected service life of the coating. At high heat, lithium oxide emits a very detectable spectral pattern, which increases in intensity along with degradation of the coating. Implementation would allow in situ monitoring of such systems, enabling an efficient means to predict lifetime until failure or necessary maintenance.
A possible new use is as a replacement for lithium cobalt oxide as the cathode in the lithium ion batteries used to power electronic devices from mobile phones to laptop computers to battery-powered cars.[6]
See also
References
- ^ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0070494398
- ^ a b Greenwood, Norman N.; Earnshaw, A. (1984). Chemistry of the Elements. Oxford: Pergamon. pp. 97–99. ISBN 0-08-022057-6.
- ^ Zintl, E.; Harder, A.; Dauth B. (1934). "Gitterstruktur der oxyde, sulfide, selenide und telluride des lithiums, natriums und kaliums". Zeitschrift für Elektrochemie und Angewandte Physikalische Chemie 40: 588–93.
- ^ Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
- ^ A spectroscopic determination of the bond length of the LiOLi molecule: Strong ionic bonding, D. Bellert, W. H. Breckenridge, J. Chem. Phys. 114, 2871 (2001); doi:10.1063/1.1349424
- ^ "Air power". The Economist, Technology Quarterly. September 3, 2009. http://www.economist.com/search/displaystory.cfm?story_id=14299690. Retrieved September 9, 2009.
External links
Lithium compounds Inorganic Organic Minerals Amblygonite · Elbaite · Eucryptite · Jadarite · Lepidolite · Lithiophilite · Petalite · Pezzottaite · Saliotite · Spodumene · Sugilite · Tourmaline · Zabuyelite · ZinnwalditeCategories:- Oxides
- Lithium compounds
- Common oxide glass components
Wikimedia Foundation. 2010.
