- Sodium oxide
-
Sodium oxide 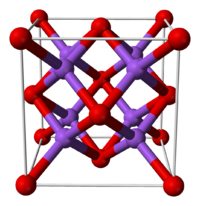
 Sodium oxideOther namesDisodium oxide
Sodium oxideOther namesDisodium oxideIdentifiers CAS number 1313-59-3 
PubChem 73971 UN number 1825 Properties Molecular formula Na2O Molar mass 61.9789 g/mol Appearance White solid Density 2.27 g/cm3 Melting point 1132°C
Boiling point 1950 °C decomposes
Solubility in water reacts violently to form NaOH Structure Crystal structure Antifluorite (face centered cubic), cF12 Space group Fm3m, No. 225 Coordination
geometryTetrahedral (Na+); cubic (O2–) Thermochemistry Std enthalpy of
formation ΔfHo298−414.2 kJ/mol Standard molar
entropy So29875.1 J mol−1 K−1 Hazards MSDS ICSC 1653 EU Index Not listed Main hazards Corrosive, reacts violently with water Flash point Non-flammable Related compounds Other anions Sodium sulfide
Sodium selenide
Sodium tellurideOther cations Lithium oxide
Potassium oxide
Rubidium oxide
Caesium oxideRelated sodium oxides Sodium peroxide
Sodium superoxideRelated compounds Sodium hydroxide  oxide (verify) (what is:
oxide (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Sodium oxide (SOX) is a chemical compound with the formula Na2O. It is used in ceramics and glasses, though not in a raw form. Treatment with water affords sodium hydroxide.
- Na2O + H2O → 2 NaOH
The alkali metal oxides M2O (M = Li, Na, K, Rb) crystallise in the antifluorite structure. In this motif the positions of the anions and cations are reversed relative to their positions in CaF2, with sodium ions tetrahedrally coordinated to 4 oxide ions and oxide cubically coordinated to 8 sodium ions.[1][2]
Contents
Preparation
Sodium oxide is produced by the reaction of sodium with sodium hydroxide, sodium peroxide, or sodium nitrite:[3]
- 2 NaOH + 2 Na → 2 Na2O + H2
- Na2O2 + 2 Na → 2 Na2O
- 2 NaNO2 + 6 Na → 4 Na2O + N2
Most of these reactions rely on the reduction of something by sodium, whether it is hydroxide, peroxide, or nitrite.
Burning sodium in air will produce Na2O and about 20% sodium peroxide Na2O2.
- 6 Na + 2 O2 → 2 Na2O + Na2O2
Applications
Glass making
Sodium oxide is a significant component of glasses and windows although it is added in the form of "soda" (sodium carbonate). Sodium oxide does not explicitly exist in glasses, since glasses are complex cross-linked polymers. Typically, manufactured glass contains around 15% sodium oxide, 70% silica (silicon dioxide) and 9% lime (calcium oxide). The sodium carbonate "soda" serves as a flux to lower the temperature at which the silica melts. Soda glass has a much lower melting temperature than pure silica, and has slightly higher elasticity. These changes arise because the silicon dioxide and soda react to form sodium silicates of the general formula Na2[SiO2]x[SiO3].
- Na2CO3 → Na2O + CO2
- Na2O + SiO2 → Na2SiO3
References
- ^ Zintl, E.; Harder, A.; Dauth B. (1934), "Gitterstruktur der oxyde, sulfide, selenide und telluride des lithiums, natriums und kaliums", Z. Elektrochem. Angew. Phys. Chem. 40: 588–93
- ^ Wells, A.F. (1984) Structural Inorganic Chemistry, Oxford: Clarendon Press. ISBN 0-19-855370-6.
- ^ Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. ISBN 0-7506-3365-4.
External links
Sodium compounds NaAlO2 · NaBH3(CN) · NaBH4 · NaBr · NaBrO3 · NaCH3COO · NaCN · NaC6H5CO2 · NaC6H4(OH)CO2 · NaCl · NaClO · NaClO2 · NaClO3 · NaClO4 · NaF · NaH · NaHCO3 · NaHSO3 · NaHSO4 · NaI · NaIO3 · NaIO4 · NaMnO4 · NaNH2 · NaNO2 · NaNO3 · NaN3 · NaOH · NaO2 · NaPO2H2 · NaReO4 · NaSCN · NaSH · NaTcO4 · NaVO3 · Na2CO3 · Na2C2O4 · Na2CrO4 · Na2Cr2O7 · Na2MnO4 · Na2MoO4 · Na2O · Na2O2 · Na2O(UO3)2 · Na2S · Na2SO3 · Na2SO4 · Na2S2O3 · Na2S2O4 · Na2S2O5 · Na2S2O6 · Na2S2O7 · Na2S2O8 · Na2Se · Na2SeO3 · Na2SeO4 · Na2SiO3 · Na2Te · Na2TeO3 · Na2Ti3O7 · Na2U2O7 · NaWO4 · Na2Zn(OH)4 · Na3N · Na3P · Na3VO4 · Na4Fe(CN)6 · Na5P3O10 · NaBiO3
Categories:- Oxides
- Sodium compounds
- Common oxide glass components
- Inorganic compound stubs
Wikimedia Foundation. 2010.
