- Sodium phosphide
-
Sodium phosphide 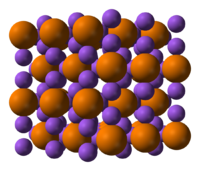 Other namessodium phosphide, common
Other namessodium phosphide, common
trisodiophosphineIdentifiers CAS number 12058-85-4 PubChem 61547 ChemSpider 55463 
Jmol-3D images Image 1 - [Na+].[Na+].[Na+].[PH6-3]
Properties Molecular formula Na3P Molar mass 99.943 g/mol Appearance black solid Solubility in water insoluble Solubility insoluble in liquid CO2 Structure Crystal structure hexagonal
a = 4.9512 Å
c = 8.7874 ÅCoordination
geometryaround P 5 near neighbours, trigonal bipyramid [1] Related compounds Other anions sodium chloride
sodium nitrideOther cations aluminium phosphide
lithium phosphide phosphide (verify) (what is:
phosphide (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Sodium phosphide, Na3P, is a black, ionic salt containing the alkali metal sodium and the phosphide anion.[2] Na3P is a source of the highly reactive phosphide anion. It should not be confused with sodium phosphate, Na3PO4.
In addition to Na3P, five other binary compositions of sodium and phosphorus are known: NaP, Na3P7, Na3P11, NaP7, and NaP15.[3]
Contents
Properties
Like K3P, solid Na3P features pentacoordinate P centers.[1] In the gas phase, molecular Na3P and Li3P are both trigonal pyramidal, with a lone pair of electrons on the central phosphorus atom.
Preparation
The first preparation of Na3P was first reported in the mid-19th century. French researcher, Alexandre Baudrimont prepared sodium phosphide by reacting molten sodium with phosphorus pentachloride.[4]
Many different routes to Na3P have been described. Due to its flammability and toxicity, Na3P (and related salts) are generally prepared in situ. White phosphorus is reduced by sodium-potassium alloy to give the phosphide salt.[5]
The conversion of white phosphorus to the phosphide has been well studied. Phosphorus reacts with sodium in an autoclave at 150 °C for 5 hours to produce Na3P. [6]
- P4 + 12 Na → 4 Na3P
Alternatively the reaction can be conducted at normal pressures but using a temperatures gradient to generate nonvolatile NaxP phases (x < 3) that then react further with sodium.[7] In some cases, an electron-transfer agent, such as naphthalene, is used. In such applications, the naphthalene forms the soluble radical anions and more rapidly reduces the phosphorus.[8]
Uses
Sodium phosphide is a source of the highly reactive phosphide anion. The material is insoluble in all solvents but reacts as a slurry with acids and related electrophiles to give derivatives of the type PM3:[5]
- Na3P + 3 M+5 → M3P (M = H, Me3Si)
The trimethylsilyl derivative is volatile (b.p. 30-35 C @ 0.001 mm Hg) and soluble. It serves as a soluble equivalent to "P3-".
Indium phosphide, a semiconductor arises by treating in-situ generated "sodium phosphide" with indium(III) chloride. In this process, the phosphide reagent is generated in situ from sodium metal and white phosphorus in N,N’-dimethylformamide, DMF.[9]
Sodium phosphide is also employed commercially as a catalyst in conjunction with zinc phosphide and aluminium phosphide for polymer production. When Na3P is removed from the ternary catalyst polymerization of propylene and 4-methyl-1-pentene is not effective.[10][citation needed]
Precautions
Sodium phosphide is highly dangerous releasing toxic phosphine upon hydrolysis, a process that is so exothermic that fires result. The USDOT has forbidden the transportation of Na3P on passenger aircraft, cargo only aircraft, and trains due to the potential fire and toxic hazards.[1]
References
- ^ a b Dong, Y; Disalvo, F.J (2005). "Reinvestigation of Na3P based on single-crystal data". Acta Crystallogr. E 61 (11): i223–i224. doi:10.1107/S1600536805031168.
- ^ Yunle, G; Fan, G; Yiate, Q; Huagui, Z; Ziping, Y (2002). "A solvothermal synthesis of ultra-fine iron phosphide". Mater. Res. Bull. 37 (6): 1101–1106. doi:10.1016/S0025-5408(02)00749-3.
- ^ Inorganic Chemistry, Egon Wiberg, Arnold Frederick Holleman Elsevier 2001 ISBN 0-12-352651-5
- ^ Baudrimont. Ann. Chim. Phys. 1864, volume 2, 13.
- ^ a b Gerd Becker, Helmut Schmidt, Gudrun Uhl “Tris(Trimethylsilyl)Phosphine and Lithium Bis(Trimethylsilyl)Phosphide.Bis-(Tetrahydrofuran)” Inorganic Syntheses, 1990, Volume 27, 243-249. doi:10.1002/9780470132586.ch48
- ^ Xie, Y; Su, H; Li, B; Qian, Y (2000). "Solvothermal preparation of tin phosphide nanorods". Mater. Res. Bull. 35 (5): 675–680. doi:10.1016/S0025-5408(00)00263-4.
- ^ Jarvis, R.F; Jacubinas, R.M; Kaner, R.B (2000). "Self-Propagating Metathesis Routes to Metastable Group 4 Phosphides". Inorg. Chem. 39 (15): 3243–3246. doi:10.1021/ic000057m. PMID 11196860.
- ^ Peterson, D.J. 1967. Patent No. 3,397,039.
- ^ Khanna, P.K; Eum, M.-S; Jun, K.-W; Baeg, J.-O; Seok, S. I (2003). "A novel synthesis of indium phosphide nanoparticles". Mater. Lett. 57 (30): 4617–4621. doi:10.1016/S0167-577X(03)00371-9.
- ^ Atarashi, Y.; Fukumoto, O. Japanese Patent No. JP 42,006,269.
External links
Sodium compounds NaAlO2 · NaBH3(CN) · NaBH4 · NaBr · NaBrO3 · NaCH3COO · NaCN · NaC6H5CO2 · NaC6H4(OH)CO2 · NaCl · NaClO · NaClO2 · NaClO3 · NaClO4 · NaF · NaH · NaHCO3 · NaHSO3 · NaHSO4 · NaI · NaIO3 · NaIO4 · NaMnO4 · NaNH2 · NaNO2 · NaNO3 · NaN3 · NaOH · NaO2 · NaPO2H2 · NaReO4 · NaSCN · NaSH · NaTcO4 · NaVO3 · Na2CO3 · Na2C2O4 · Na2CrO4 · Na2Cr2O7 · Na2MnO4 · Na2MoO4 · Na2O · Na2O2 · Na2O(UO3)2 · Na2S · Na2SO3 · Na2SO4 · Na2S2O3 · Na2S2O4 · Na2S2O5 · Na2S2O6 · Na2S2O7 · Na2S2O8 · Na2Se · Na2SeO3 · Na2SeO4 · Na2SiO3 · Na2Te · Na2TeO3 · Na2Ti3O7 · Na2U2O7 · NaWO4 · Na2Zn(OH)4 · Na3N · Na3P · Na3VO4 · Na4Fe(CN)6 · Na5P3O10 · NaBiO3
Categories:- Phosphides
- Sodium compounds
Wikimedia Foundation. 2010.
