- Sodium molybdate
-
Sodium molybdate 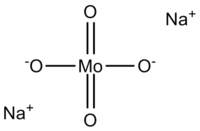
 Sodium molybdateOther namesDisodium molybdate
Sodium molybdateOther namesDisodium molybdateIdentifiers CAS number 7631-95-0  ,
,
10102-40-6 (dihydrate)PubChem 4384450 EC number 231-551-7 RTECS number QA5075000 Properties Molecular formula Na2MoO4 Molar mass 205.92 g/mol (anhydrous)
241.95 g/mol (dihydrate)Appearance White powder Density 3.78 g/cm3, solid Melting point 687 °C
Solubility in water 84 g/100 ml (100 °C) Refractive index (nD) 1.714 Hazards MSDS External MSDS EU Index Not listed NFPA 704 Flash point Non-flammable Related compounds Other anions Sodium chromate
Sodium tungstateOther cations Ammonium molybdate  molybdate (verify) (what is:
molybdate (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Sodium molybdate, Na2MoO4, is useful as a source of molybdenum.[1] It is often found as the dihydrate, Na2MoO4·2H2O.
The molybdate(VI) anion is tetrahedral. Two sodium cations coordinate with every one anion.[2]
Contents
History
Sodium molybdate was first synthesized by the method of hydration.[3] A more convenient synthesis is done by dissolving MoO3 in sodium hydroxide at 50–70 °C and crystallizing the filtered product.[2] The anhydrous salt is prepared by heating to 100 °C.
- MoO3 + 2NaOH → Na2MoO4·2H2O
Uses
The agriculture industry uses 1 million pounds per year as a fertilizer. In particular, its use has been suggested for treatment of whiptail in broccoli and cauliflower in molybdenum-deficient soils.[4][5] However, care must be taken because at a level of 0.3 ppm sodium molybdate can cause copper deficiencies in animals, particularly cattle.[2]
It is used in industry for corrosion inhibition, as it is a non-oxidizing anodic inhibitor.[2] The addition of sodium molybdate significantly reduces the nitrite requirement of fluids inhibited with nitrite-amine, and improves the corrosion protection of carboxylate salt fluids.[6]
In industrial water treatment applications where galvanic corrosion is a potential due to bimetallic construction, the application of sodium molybdate is preferred over sodium nitrite. Sodium molybdate has the advantage in that the dosing of lower ppm's of molybdate allow for lower conductivity of the circulating water. Sodium molybdate at levels of 50-100 ppm offer the same levels of corrosion inhibition that sodium nitrite at levels of 800+ offer. By utilizing lower concentrations of sodium molybdate, conductivity is kept at a minimum and thus galvanic corrosion potentials are decreased.[7]
Reactions
When reacted with sodium borohydride, molybdenum is reduced to a lower valent oxide:[8]
- Na2MoO4 + NaBH4 + 2H2O→ NaBO2 + MoO2 + 2NaOH+ 3 H2
Sodium molybdate reacts with the acids of dithiophosphates:[2]
- Na2MoO4 + (RO)2PS2H (R = Me, Et) → [MoO2(S2P(OR)2)2]
which further reacts to form [MoO3(S2P(OR)2)4].
Safety
Sodium molybdate is incompatible with alkali metals, most common metals and oxidizing agents. It will explode on contact with molten magnesium. It will violently react with interhalogens (e.g., bromine pentafluoride; chlorine trifluoride). Its reaction with hot sodium, potassium or lithium is incandescent.[9]
References
- ^ Greenwood, Norman N.; Earnshaw, A. (1984). Chemistry of the Elements. Oxford: Pergamon. ISBN 0-08-022057-6.
- ^ a b c d e Braithwaite, E.R.; Haber, J. Molybdenum: An outline of its Chemistry and Uses. 1994. Elsevier Science B.V. Amsterdam, The Netherlands.
- ^ Spitsyn, Vikt. I.; Kuleshov, I. M. Zhurnal Obshchei Khimii 1951. 21. 1701-15.
- ^ Plant, W. (1950). "Use of Lime and Sodium Molybdate for the Control of ‘Whiptail’ in Broccoli". Nature 165 (4196): 533. doi:10.1038/165533b0.
- ^ Davies, E. B. (1945). "A Case of Molybdenum Deficiency in New Zealand". Nature 156 (3961): 392. doi:10.1038/156392b0.
- ^ Vukasovich, Mark S. Lubrication Engineering 1980. 36(12). 708-12.
- ^ M. Houser, Corrosion Control Services, Inc., Introduction Handbook
- ^ Chi Fo Tsang and Arumugam Manthiram. Journal of Materials Chemistry 1997. 7(6). 1003–1006.
- ^ http://www.mallbaker.com/americas/msds/english/s4394_msds_us_default.pdf
External links
Sodium compounds NaAlO2 · NaBH3(CN) · NaBH4 · NaBr · NaBrO3 · NaCH3COO · NaCN · NaC6H5CO2 · NaC6H4(OH)CO2 · NaCl · NaClO · NaClO2 · NaClO3 · NaClO4 · NaF · NaH · NaHCO3 · NaHSO3 · NaHSO4 · NaI · NaIO3 · NaIO4 · NaMnO4 · NaNH2 · NaNO2 · NaNO3 · NaN3 · NaOH · NaO2 · NaPO2H2 · NaReO4 · NaSCN · NaSH · NaTcO4 · NaVO3 · Na2CO3 · Na2C2O4 · Na2CrO4 · Na2Cr2O7 · Na2MnO4 · Na2MoO4 · Na2O · Na2O2 · Na2O(UO3)2 · Na2S · Na2SO3 · Na2SO4 · Na2S2O3 · Na2S2O4 · Na2S2O5 · Na2S2O6 · Na2S2O7 · Na2S2O8 · Na2Se · Na2SeO3 · Na2SeO4 · Na2SiO3 · Na2Te · Na2TeO3 · Na2Ti3O7 · Na2U2O7 · NaWO4 · Na2Zn(OH)4 · Na3N · Na3P · Na3VO4 · Na4Fe(CN)6 · Na5P3O10 · NaBiO3
Categories:- Molybdates
- Sodium compounds
- Dietary minerals
- Inorganic compounds
Wikimedia Foundation. 2010.


