- Nifedipine
-
Nifedipine 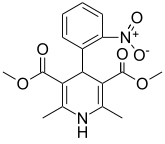
Systematic (IUPAC) name 3,5-dimethyl 2,6-dimethyl-4-(2-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate Clinical data Trade names Procardia AHFS/Drugs.com monograph MedlinePlus a684028 Pregnancy cat. C: (USA) Legal status ? Routes Oral Pharmacokinetic data Bioavailability 45-56% Protein binding 92-98% Metabolism Gastrointestinal, Hepatic Half-life 2 hours Excretion Renal: >50%, Biliary: 5-15% Identifiers CAS number 21829-25-4 
ATC code C08CA05 PubChem CID 4485 DrugBank APRD00590 ChemSpider 4330 
UNII I9ZF7L6G2L 
KEGG D00437 
ChEBI CHEBI:7565 
ChEMBL CHEMBL193 
Chemical data Formula C17H18N2O6 Mol. mass 346.335 g/mol SMILES eMolecules & PubChem Physical data Melt. point 173 °C (343 °F)  (what is this?) (verify)
(what is this?) (verify)Nifedipine (brand names Adalat, Nifediac, Cordipin, Nifedical, and Procardia) is a dihydropyridine calcium channel blocker. Its main uses are as an antianginal (especially in Prinzmetal's angina) and antihypertensive, although a large number of other indications have recently been found for this agent, such as Raynaud's phenomenon, premature labor, and painful spasms of the esophagus in cancer and tetanus patients. It is also commonly used for the small subset of pulmonary hypertension patients whose symptoms respond to calcium channel blockers.
Contents
Dosing
The recommended starting dose for immediate-release nifedipine capsules is 10 mg, taken 3 times daily. With the extended-release version, the recommended starting nifedipine dosage is 30 to 60 mg, taken once daily. Nifedipine rapidly lowers blood pressure, and patients are commonly warned they may feel dizzy or faint after taking the first few doses. Tachycardia (fast heart rate) may occur as a reaction. These problems are much less frequent in the sustained-release preparations of nifedipine (such as Adalat OROS). A more novel release system is GITS (Gastro-Intestinal Therapeutic System), which - according to Bayer - provides 24-hour continuous release through an osmotic push system. Recent trials with GITS include INSIGHT (for blood pressure)[1] and ACTION (for angina).[2]
Extended release formulations of nifedipine should be taken on an empty stomach, and patients are warned not to consume anything containing grapefruit or grapefruit juice, as they raise blood nifedipine levels. There are several possible mechanisms, including the lowering of CYP3A4 activity.[3]
Uses
Approved uses
The approved uses for nifedipine are the long-term treatment of hypertension (high blood pressure) and angina pectoris. In hypertension, recent clinical guidelines generally favour diuretics and ACE inhibitors, although calcium channel antagonists, along with thiazide diuretics, are still favoured as primary treatment for over 55's and black patients.[4]
Sublingual nifedipine has previously been used in hypertensive emergencies. This was found to be dangerous, and has been abandoned. Sublingual nifedipine causes blood-pressure lowering through peripheral vasodilation. It can cause an uncontrollable decrease in blood pressure, reflex tachycardia, and a steal phenomenon in certain vascular beds. There have been multiple reports in the medical literature of serious adverse effects with sublingual nifedipine, including cerebral ischemia/infarction, myocardial infarction, complete heart block, and death. As a result of this, the FDA reviewed all data regarding the safety and efficacy of sublingual nifedipine for hypertensive emergencies in 1995, and concluded that the practice should be abandoned because it was neither safe nor efficacious. [5][6] An exception to the avoidance of this practice is in the use of nifedipine in the treatment of hypertension associated with autonomic dysreflexia in spinal cord injury.[7]
Off-label uses
Nifedipine has been used frequently as a tocolytic (agent that delays premature labor). A Cochrane review has concluded that it is comparable with magnesium sulfate and beta-agonists (such as ritodrine) with fewer side-effects.[8] Its role vis à vis atosiban is not established.
Raynaud's phenomenon is often treated with nifedipine. A 2005 meta-analysis showed modest benefits (33% decrease in attack severity, 2.8-5 reduction in absolute number of attacks per week); it does conclude that most included studies used low doses of nifedipine.[9]
Topical nifedipine has been shown to be as effective as topical nitrates for anal fissures.[10]
Nifedipine is also used in high-altitude medicine to treat high altitude pulmonary edema.[11]
Oral nifedipine has also been found to cause iron loss in the urine of small animals.[12] A NIH NIDDK study[13] is currently seeing if the drug can increase the removal of iron into the urine in humans as well, thus becoming a possible treatment for iron overload.
Overdosage
A number of persons have developed toxicity due to acute overdosage with nifedipine, either accidentally or intentionally, and via either oral or parenteral administration. The adverse effects include lethargy, bradycardia, marked hypotension and loss of consciousness. The drug may be quantitated in blood or plasma to confirm a diagnosis of poisoning in hospitalized patients or to assist in a medicolegal death investigation. Analytical methods usually involve gas or liquid chromatography and specimen concentrations are usually in the 100-1000 μg/L range.[14][15]
History
Nifedipine (initially BAY a1040) was developed by the German pharmaceutical company Bayer, with most initial studies being performed in the early 1970s.[16]
The use of nifedipine and related calcium channel antagonists was much reduced in response to 1995 trials that mortality was increased in patients with coronary artery disease who took nifedipine.[17] This study was a meta-analysis, and demonstrated harm mainly in short-acting forms of nifedipine (that could cause large fluctations in blood pressure) and at high doses of 80 mg a day and more.[18]
References
- ^ Brown MJ, Palmer CR, Castaigne A, et al. (2000). "Morbidity and mortality in patients randomised to double-blind treatment with a long-acting calcium-channel blocker or diuretic in the International Nifedipine GITS study: Intervention as a Goal in Hypertension Treatment (INSIGHT)". Lancet 356 (9227): 366–72. doi:10.1016/S0140-6736(00)02527-7. PMID 10972368.
- ^ Poole-Wilson PA, Kirwan BA, Vokó Z, de Brouwer S, van Dalen FJ, Lubsen J (2006). "Safety of nifedipine GITS in stable angina: the ACTION trial". Cardiovas Drugs Ther 20 (1): 45–54. doi:10.1007/s10557-006-6312-4. PMID 16552473.
- ^ Odou P, Ferrari N, Barthélémy C, et al. (2005). "Grapefruit juice-nifedipine interaction: possible involvement of several mechanisms". Journal Clinical Pharm Ther 30 (2): 153–8. doi:10.1111/j.1365-2710.2004.00618.x. PMID 15811168.
- ^ Hypertension: management of hypertension in adults in primary care. Clinical guideline CG34. National Institute for Health and Clinical Excellence (NICE), June 2006. Fulltext index. ISBN 1-86016-285-1.
- ^ Grossman E, Messerli FH, Grodzicki T, Kowey P (1996). "Should a moratorium be placed on sublingual nifedipine capsules given for hypertensive emergencies and pseudoemergencies?". JAMA 276 (16): 1328–31. doi:10.1001/jama.276.16.1328. PMID 8861992.
- ^ Varon J, Marik PE (2003). "Clinical review: The management of hypertensive crises". Critical care (London, England) 7 (5): 374–84. doi:10.1186/cc2351. PMC 270718. PMID 12974970. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=270718.
- ^ Campagnolo, DI. "Autonomic Dysreflexia in Spinal Cord Injury". eMedicine. http://emedicine.medscape.com/article/322809-medication. Retrieved 7/14/11.
- ^ King JF, Flenady VJ, Papatsonis DN, Dekker GA, Carbonne B (2003). Flenady, Vicki. ed. "Calcium channel blockers for inhibiting preterm labour". Cochrane database of systematic reviews (Online) (1): CD002255. doi:10.1002/14651858.CD002255. PMID 12535434.
- ^ Thompson AE, Pope JE (2005). "Calcium channel blockers for primary Raynaud's phenomenon: a meta-analysis". Rheumatology (Oxford, England) 44 (2): 145–50. doi:10.1093/rheumatology/keh390. PMID 15546967.
- ^ Ezri T, Susmallian S (2003). "Topical nifedipine vs. topical glyceryl trinitrate for treatment of chronic anal fissure". Dis. Colon Rectum 46 (6): 805–8. doi:10.1007/s10350-004-6660-8. PMID 12794583.
- ^ Ali, Mir Omar and Qazi, Samia (2007-09-19). "Pulmonary Edema, High-Altitude". eMedicine. http://www.emedicine.com/med/topic1956.htm. Retrieved 2007-11-25.
- ^ Ludwiczek S, Theurl I, Muckenthaler MU, Jakab M, Mair SM, Theurl M, Kiss J, Paulmichl M, Hentze MW, Ritter M, Weiss G. Ca2+ channel blockers reverse iron overload by a new mechanism via divalent metal transporter-1. Nat Med. 2007 Apr;13(4):448-54. Epub 2007 Feb 11.
- ^ http://clinicaltrials.gov/ct2/show/NCT00712738?term=iron+overload&rank=1
- ^ Nifediac package insert, TEVA Pharmaceuticals, Sellersville, Pennsylvania, August, 2009.
- ^ Baselt, Randall C. (2008). Disposition of Toxic Drugs and Chemicals in Man. Foster City, CA: Biomedical Publications. pp. 1108–1110. ISBN 0-9626523-7-7.
- ^ Vater W, Kroneberg G, Hoffmeister F, et al. (1972). "[Pharmacology of 4-(2'-nitrophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylic acid dimethyl ester (Nifedipine, BAY a 1040)]" (in German). Arzneimittel-Forschung 22 (1): 1–14. PMID 4622472.
- ^ Furberg CD, Psaty BM, Meyer JV (1995). "Nifedipine. Dose-related increase in mortality in patients with coronary heart disease". Circulation 92 (5): 1326–31. PMID 7648682.
- ^ Opie LH, Messerli FH (1995). "Nifedipine and mortality. Grave defects in the dossier". Circulation 92 (5): 1068–73. PMID 7648646.
External links
Tocolytics/labor repressants (G02CA) β2-agonists Oxytocin antagonist NSAID Calcium channel blocker NifedipineMyosin inhibitor Categories:- Calcium channel blockers
- Tocolytics
- Dihydropyridines
- Carboxylate esters
- Nitrobenzenes
Wikimedia Foundation. 2010.
