- Magnesium citrate
-
Magnesium citrate 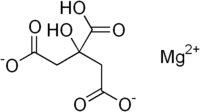 Magnesium 2-hydroxypropane-1,2,3-tricarboxylate
Magnesium 2-hydroxypropane-1,2,3-tricarboxylateIdentifiers CAS number 7779-25-1 
PubChem 24511 EC number 231-923-9 KEGG D03265 
Jmol-3D images Image 1 - C(C(=O)O)C(CC(=O)[O-])(C(=O)[O-])O.[Mg+2]
Properties Molecular formula C6H6MgO7 Molar mass 214.41 g mol−1 Related compounds Related salts Trimagnesium citrate  citrate (verify) (what is:
citrate (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Magnesium citrate, a magnesium salt of citric acid, is a chemical agent used medicinally as a saline laxative and to completely empty the bowel prior to a major surgery or colonoscopy. It is available without a prescription, both as a generic brand or under the brand name Citromag or Citroma. It is also used as a magnesium supplement in pills. The magnesium content of magnesium citrate corresponds to about 11% by mass.
Contents
Mechanism of action
Magnesium citrate works by attracting water through the tissues by a process known as osmosis. Once in the intestine, it can attract enough water into the intestine to induce defecation. The additional water helps to create more feces, which naturally stimulates bowel motility. This means it can also be used to treat rectal and colon problems. Magnesium citrate functions best on an empty stomach, and should always be followed with a full (eight ounce) glass of water or juice to help the magnesium citrate absorb properly and help prevent any complications. Magnesium citrate is generally not a harmful substance, but care should be taken by consulting a health-care professional if any adverse health problems are suspected or experienced.
Use and dosage
The maximum Upper Tolerable Limit for magnesium in supplement form for adults is 350 mg per day of elemental magnesium according to the National Institutes of Health (NIH).[1] In addition, according to the NIH, total dietary requirements for magnesium from all sources (i.e. food and supplements) is 320–420 mg of elemental magnesium per day, though there is no UTL for dietary Magnesium. As a laxative syrup with a concentration of 1.745 g of magnesium citrate per fl. oz, a typical dose for adults and children twelve years or older is between 7 and 10 US fluid ounces (210 and 300 ml; 7.3 and 10 imp fl oz), followed immediately with a full 8 US fluid ounces (240 ml; 8.3 imp fl oz) glass of water. Consuming an adult dose of 10 oz of laxative syrup (@ 1.745 g/oz) implies a consumption of 17.45 g of magnesium citrate in a single 10 oz dose resulting in a consumption of approximately 2.0 g of elemental magnesium per single dose. Given that this laxative dose contains six times the normal nutritional dose for magnesium, caution should be taken to avoid prolonged usage (i.e. over five days) and to follow the manufacturer's instructions strictly.[citation needed] For children between three and twelve years of age, the typical dose is roughly half that,[citation needed] based on physician recommendation. Magnesium citrate is not recommended for use in children and infants two years of age or less.[2]
Although less common, as a magnesium supplement the citrate form is sometimes used due to its increased bio-availability to other common pill forms, such as magnesium oxide.[citation needed] However, according to some studies magnesium gluconate may be more bio-available than magnesium citrate.[3] Higher doses, up to 500 mg daily, have been used effectively in the prophylaxis of migraines, in combination with riboflavin (vitamin B2) 400 mg and, in some cases, a supplement of coenzyme Q10.[citation needed] Similar dosages apply when used as a supplement to help prevention of kidney stones.[citation needed]
Magnesium citrate, as a supplement in pill form, is useful for the prevention of kidney stones.[4]
Side effects
It is always important to correctly follow the prescribed doses; extreme magnesium overdose can result in serious complication such as slow heart beat, low blood pressure, nausea, drowsiness, etc. If severe enough, an overdose can even result in coma or death.[5] However, a moderate overdose will be excreted through the kidneys, unless one suffers from serious kidney problems.
Magnesium citrate solutions generally produces bowel movement in one half to six hours. Rectal bleeding or failure to have a bowel movement after use could be signs of a serious condition.
See also
- Trimagnesium citrate A more alkaline version of this salt, with 50% more magnesium. It is also available through pill or tablet form if needed.
- ATC code A12
References
- ^ Magnesium. Office of Dietary Supplements (ODS). National Institutes of Health (NIH).
- ^ "magnesium_citrate-oral" at medicinenet.com
- ^ Coudray, C; Rambeau, M; Feillet-Coudray, C; Gueux, E; Tressol, JC; Mazur, A; Rayssiguier, Y (December 2005). "Study of magnesium bioavailability from ten organic and inorganic Mg salts in Mg-depleted rats using a stable isotope approach". Magnes Res 18 (4): 215–23. PMID 16548135.
- ^ Ettinger, B; Pak, CY; Citron, JT; Thomas, C; Adams-Huet, B; Vangessel, A (December 1997). "Potassium-magnesium citrate is an effective prophylaxis against recurrent calcium oxalate nephrolithiasis". J Urol 158 (6): 2069–73. doi:10.1016/S0022-5347(01)68155-2. PMID 9366314.
- ^ magnesium citrate. Cerner Multum. Drugs.com. 12 April 2009.
External links
- Saline laxatives. MedicineNet.
- Magnesium citrate Patient Advice. Drugs.com.
Magnesium compounds Mineral supplements (A12) Calcium Potassium Sodium Zinc Magnesium Magnesium chloride • Magnesium sulfate • Magnesium gluconate • Magnesium citrate • Magnesium aspartate • Magnesium lactate • Magnesium levulinate • Magnesium pidolate • Magnesium orotate • Magnesium oxideFluoride Selenium M: NUT
cof, enz, met
noco, nuvi, sysi/epon, met
drug(A8/11/12)
Categories:- Citrates
- Laxatives
- Magnesium compounds
Wikimedia Foundation. 2010.
