- Magnesium chloride
-
Magnesium chloride 
 Magnesium chlorideOther namesMagnesium dichloride
Magnesium chlorideOther namesMagnesium dichlorideIdentifiers CAS number 7786-30-3  ,
,
7791-18-6 (hexahydrate)PubChem 24584 ChemSpider 22987 
ChEBI CHEBI:6636 
ChEMBL CHEMBL1200547 
RTECS number OM2975000 Jmol-3D images Image 1 - [Mg+2].[Cl-].[Cl-]
Properties Molecular formula MgCl2 Molar mass 95.211 g/mol (anhydrous)
203.31 g/mol (hexahydrate)Appearance white or colourless crystalline solid Density 2.32 g/cm3 (anhydrous)
1.569 g/cm3 (hexahydrate)Melting point 714 °C (987 K) (on rapid heating: slow heating leads to decomposition from 300 °C)
Boiling point 1412 °C (1685 K)
Solubility in water anhydrous
54.3 g/100 ml (20 °C)
72.6 g/100 mL (100 °C)
hexahydrate
157 g/100 mL (20 °C)Solubility in ethanol 7.4 g/100 mL (30 °C) Refractive index (nD) 1.675 (anhydrous)
1.569 (hexahydrate)Structure Crystal structure CdCl2 Coordination
geometry(octahedral, 6-coordinate) Hazards MSDS ICSC 0764 EU Index Not listed R-phrases R36, R37, R38 S-phrases S26, S37, S39 Main hazards Irritant Flash point Non-flammable LD50 2800 mg/kg-1 (oral, rat) Related compounds Other anions Magnesium fluoride
Magnesium bromide
Magnesium iodideOther cations Beryllium chloride
Calcium chloride
Strontium chloride
Barium chloride
Radium chloride chloride (verify) (what is:
chloride (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Magnesium chloride is the name for the chemical compounds with the formulas MgCl2 and its various hydrates MgCl2(H2O)x. These salts are typical ionic halides, being highly soluble in water. The hydrated magnesium chloride can be extracted from brine or sea water. Magnesium chloride as the natural mineral bischofite is also extracted (solution mining) out of ancient seabeds; for example, the Zechstein seabed in northwest Europe. Anhydrous magnesium chloride is the principal precursor to magnesium metal, which is produced on a large scale. Hydrated magnesium chloride is the form usually used in prescription oral magnesium supplements.
Contents
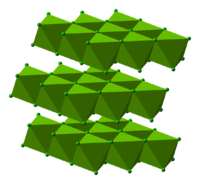
Structure, preparation, and general properties
MgCl crystallizes in the cadmium chloride motif, which features octahedral Mg. A variety of hydrates are known with the formula MgCl(H2O)x, and each loses water with increasing temperature: x = 12 (-16.4 °C), 8 (-3.4 °C), 6 (116.7 °C), 4 (181 °C), 2 (ca. 300 °C).[1] In the hexahydrate, the Mg+ remains octahedral, but is coordinated to six water ligands.[2] The thermal dehydration of the hydrates MgCl(H2O)x (x = 6, 12) does not occur straightforwardly.[3]
As suggested by the existence of some hydrates, anhydrous MgCl2 is a Lewis acid, although a very weak one.
In the Dow process, magnesium chloride is regenerated from magnesium hydroxide using hydrochloric acid:
It can also be prepared from magnesium carbonate by a similar reaction.
In most of its derivatives, MgCl forms octahedral complexes. Derivatives with tetrahedral Mg2+ are less common. Examples include salts of (tetraethylammonium)2MgCl4 and adducts such as MgCl(TMEDA).[4]
Applications
Magnesium chloride serves as precursor to other magnesium compounds, for example by precipitation:
It can be electrolysed to give magnesium metal:[5]
This process is practiced on a substantial scale.
Magnesium chloride is used for a variety of other applications besides the production of magnesium: the manufacture of textiles, paper, fireproofing agents, cements and refrigeration brine,[5] and dust and erosion control. Mixed with hydrated magnesium oxide, magnesium chloride forms a hard material called Sorel cement.
Magnesium ion Mg2+ (usually added as the chloride) is an important component in the polymerase chain reaction, a procedure used to amplify DNA fragments. It is generally used in experimental biology whenever RNA and DNA and their enzymes are to function in vitro, since Mg2+ is a necessary associate ion for nucleotides in biology, such as ATP.
Magnesium chloride is also used in several medical and topical (skin related) applications. It has been used in pills as supplemental sources of magnesium, where it serves as a soluble compound which is not as laxative as magnesium sulfate, and more bioavailable than magnesium hydroxide and magnesium oxide, since it does not require stomach acid to produce soluble Mg2+ ion. It can also be used as an effective anaesthetic for cephalopods, some species of crustaceans,[6] and several species of bivalve, including oysters.[7]
Culinary use
Magnesium chloride (E511[8]) is an important coagulant used in the preparation of tofu from soy milk. In Japan it is sold as nigari (にがり, derived from the Japanese word for "bitter"), a white powder produced from seawater after the sodium chloride has been removed, and the water evaporated. In China it is called lushui (卤水). Nigari or lushui consists mostly of magnesium chloride, with some magnesium sulfate and other trace elements. It is also an ingredient in baby formula milk.
Use as an anti-icer
A number of state highway departments throughout the United States have decreased the use of rock salt and sand on roadways and have increased the use of solutions of magnesium chloride (often called "liquid magnesium chloride") as a de-icer or anti-icer. Magnesium chloride is much less toxic to plant life surrounding highways and airports, and is less corrosive to concrete and steel (and other iron alloys) than sodium chloride. The liquid magnesium chloride is sprayed on dry pavement (tarmac) prior to precipitation or wet pavement prior to freezing temperatures in the winter months to prevent snow and ice from adhering and bonding to the roadway. The application of anti-icers is utilized in an effort to improve highway safety. Magnesium chloride is also sold in crystal form for household and business use to de-ice sidewalks and driveways. In these applications, the compound is applied after precipitation has fallen or ice has formed, instead of previously.
The use of this compound seems to show an improvement in driving conditions during and after freezing precipitation, but it can damage electric utilities. This occurs in two ways: contamination of insulators, causing tracking and arcing across them, and corrosion of steel and aluminium poles and pole hardware.
Use in dust and erosion control
Road departments and private industry may apply liquid or powdered magnesium chloride to control dust and erosion on unimproved (dirt or gravel) roads and dusty job sites such as quarries. Its hygroscopy makes it absorb moisture from the air, controlling the number of small particles which become airborne. Owners of indoor arenas (e.g. for horse riding) may apply magnesium chloride to sand or other floor materials to control dust.
Use in hydrogen storage
Magnesium chloride has shown promise as a storage material for hydrogen. Ammonia, which is rich in hydrogen atoms, is used as an intermediate storage material. Ammonia can be effectively absorbed onto solid magnesium chloride, forming Mg(NH3)6Cl. Ammonia is released by mild heat, and is then passed through a catalyst to give hydrogen gas.
Medical and veterinary use
Medically-prescribed magnesium supplements such as Slo-Mag and Mag-SR contain magnesium chloride which is slowly released from a matrix. However, since magnesium is absorbed by the body in ionic form (after the salt dissolves in water) such supplements have no advantage over any soluble magnesium salt (for example, magnesium citrate or magnesium aspartate).
One veterinary study in 1989 indicated some effectiveness against tumors when magnesium chloride was used as a feed additive.[9]
Experimental antilipid effects in animals
In a recent experiment with mice, MgCl fortification of drinking water reduced cholesterol and triglyceride levels, although overall plasma lipid levels were similar at the beginning and the end of the study.[10]
Marine aquarium use
Magnesium in natural seawater values are between 1250 mg/L and 1350 mg/L. Magnesium helps to stabilize the correct combination of calcium, alkalinity, and pH values. Severely low values of magnesium (900 mg/L or below) can cause low pH values and an inability to maintain proper alkalinity and calcium values. If levels of magnesium become too low, coral growth ceases; decline of coral health follows. Carbonates and calcium are essential for all growth of corals, coralline algae, clams, and invertebrates. Maintaining the correct magnesium values is very important and is indirectly responsible for coral and coralline algae growth by making it possible to maintain correct calcium, alkalinity, and pH values. Magnesium can be depleted by mangrove plants and the use of excessive Kalkwasser or by going beyond natural calcium, alkalinity, and pH values.[11]
Toxicology
Magnesium ions are bitter-tasting, and magnesium chloride solutions are bitter in varying degrees, depending on the concentration of magnesium.
Magnesium toxicity from magnesium salts is rare in healthy individuals with a normal diet, because excess magnesium is readily excreted in urine by the kidneys. A few cases of oral magnesium toxicity have been described in persons with normal renal function ingesting large amounts of magnesium salts, but it is rare. If a large amount of magnesium chloride is eaten, it will have effects similar to magnesium sulfate, causing diarrhea, although the sulfate also contributes to the laxative effect in magnesium sulfate, so the effect from the chloride is not as severe.
See also
Notes & references
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ^ Wells, A. F. (1984) Structural Inorganic Chemistry, Oxford: Clarendon Press. ISBN 0-19-855370-6.
- ^ see notes in Rieke, R. D.; Bales, S. E.; Hudnall, P. M.; Burns, T. P.; Poindexter, G S. “Highly Reactive Magnesium for the Preparation of Grignard Reagents: 1-Norbornane Acid” Organic Syntheses, Collected Volume 6, p.845 (1988). http://www.orgsyn.org/orgsyn/pdfs/CV6P0845.pdf
- ^ N. N. Greenwood, A. Earnshaw, Chemistry of the Elements, Pergamon Press, 1984.
- ^ a b Hill, Petrucci, McCreary, Perry, "General Chemistry", 4th ed., Pearson/Prentice Hall, Upper Saddle River, New Jersey, USA.
- ^ http://webs.lander.edu/rsfox/invertebrates/homarus.html
- ^ Culloty, S.C. & Mukahy, M.F. 1992. An evaluation of anaesthetics for Ostrea edulis (L.). Aquaculture. 107: 249-252.
- ^ Food Standard Agency. "Current EU approved additives and their E Numbers". http://www.food.gov.uk/safereating/chemsafe/additivesbranch/enumberlist. Retrieved 22 March 2010.
- ^ Veterinary Medical Research Institute, Iowa State University
- ^ Atherogenesis inhibition induced by magnesium-chloride fortification of drinking water
- ^ http://www.advancedaquarist.com/issues/oct2003/chem.htm
- Handbook of Chemistry and Physics, 71st edition, CRC Press, Ann Arbor, Michigan, 1990.
External links
Magnesium compounds Categories:- Chlorides
- Magnesium compounds
- Metal halides
- Deliquescent substances
Wikimedia Foundation. 2010.


