- Methoxetamine
-
Not to be confused with methoxamine.
Methoxetamine 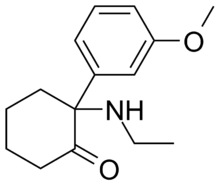
Systematic (IUPAC) name (RS)2-(3-methoxyphenyl)-2-(ethylamino)cyclohexanone Clinical data Pregnancy cat. ? Legal status ? Identifiers CAS number 1239943-76-0 
ATC code None ChemSpider 24721792 
Chemical data Formula C15H21NO2 Mol. mass 247.33 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Methoxetamine (MXE) or 3-MeO-2-Oxo-PCE is a chemical of the arylcyclohexylamine class which has been sold as a designer drug.[1] It is a derivative of ketamine that also contains structural features of eticyclidine and 3-MeO-PCP. Methoxetamine is thought to behave as a NMDA receptor antagonist and dopamine reuptake inhibitor, though it has not been formally profiled pharmacologically.[2] Methoxetamine differs from many other dissociative anesthetics of the arylcyclohexylamine class in that it was designed for grey-market distribution.[3] Methoxetamine is a product of rational drug design: its N-ethyl group was chosen to increase potency, lessening the risk of interstitial cystitis that can result from the accumulation of ketamine-like metabolites in the urinary bladder.[3][4]
See also
- Dissociatives
- Ketamine
- Phencyclidine
- Methoxydine
References
- ^ EMCDDA Annual Report 2010
- ^ Ward, J.; Rhyee, S.; Plansky, J. (2011). "Methoxetamine: A novel ketamine analog and growing health-care concern". Clinical Toxicology: 1. doi:10.3109/15563650.2011.617310.
- ^ a b [1], Morris, H. (11 February 2011). "Interview with a ketamine chemist: or to be more precise, an arylcyclohexylamine chemist". Vice Magazine. Retrieved 2011-02-11.
- ^ "4-Amino-4-arylcyclohexanones and Their Derivatives, a Novel Class of Analgesics. 1. Modification of the Aryl Ring." Daniel Lednicer, Philip F. VonVoigtlander and D. Edward Emmert. The Upjohn Company, Research Laboratories, Kalamazoo, Michigan 49001. Received August 7, 1979. J. Med. Chem 1980, 23, p424-430
External links
Categories:- Amines
- Ketones
Wikimedia Foundation. 2010.
