- Pantoprazole
-
Pantoprazole 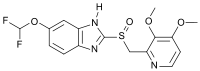
Systematic (IUPAC) name (RS)-6-(difluoromethoxy)-2-[(3,4-dimethoxypyridin-2-yl)methylsulfinyl]-1H-benzo[d]imidazole Clinical data Trade names Protonix AHFS/Drugs.com monograph MedlinePlus a601246 Licence data US FDA:link Pregnancy cat. B3(AU) B(US) Legal status ℞ Prescription only Routes Oral and intravenous Pharmacokinetic data Bioavailability 77% Metabolism Hepatic (CYP2C19 and 3A4) Half-life 1 hour Excretion Renal Identifiers CAS number 102625-70-7 
ATC code A02BC02 PubChem CID 4679 DrugBank APRD00073 ChemSpider 4517 
UNII D8TST4O562 
KEGG D05353 
ChEBI CHEBI:7915 
ChEMBL CHEMBL1502 
Chemical data Formula C16H15F2N3O4S Mol. mass 383.371 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Pantoprazole (sold under various brand names including Somac, Pantoloc, Protium, Pantecta, Protonix, and Pantoheal) is a proton pump inhibitor drug that inhibits gastric acid secretion.
Contents
Use
Pantoprazole is used for short-term treatment of erosion and ulceration of the esophagus caused by gastroesophageal reflux disease. Initial treatment is generally of eight weeks' duration, after which another eight week course of treatment may be considered if necessary.[citation needed] It can be used as a maintenance therapy for long term use after initial response is obtained.
This medication may affect the results of certain lab tests, such as drug screenings (pantoprazole can cause a false positive for THC, the psychoactive component of cannabis).[citation needed]
Pharmacology
Pantoprazole is metabolized in the liver by the cytochrome P450 system.[1] Metabolism mainly consists of demethylation by CYP2C19 followed by sulfation. Another metabolic pathway is oxidation by CYP3A4. Pantoprazole metabolites are not thought to have any pharmacological significance. Pantoprazole is relatively free of drug interactions;[2] however, it may alter the absorption of other medications that depend on the amount of acid in the stomach, such as ketoconazole or digoxin. Generally inactive at acidic pH of stomach, thus it is usually given with a pro kinetic drug. Pantoprazole binds irreversibly to H+K+ATPase(Proton pumps) and suppresses the secretion of acid. As it binds irreversibly to the pumps, new pumps have to be made before acid production could be resumed. Half life of the drug is approx 24 hours.
Availability
Pantoprazole was developed by Altana (owned by Nycomed) and was licensed in the USA to Wyeth (which was taken over by Pfizer) and is currently marketed under the brand name Protonix by Wyeth-Ayerst Laboratories, Pantec by Concept Pharmaceuticals, Somac by Pfizer, API by Vanguard Therapeutics, Astropan by Astron Lifesciences, Fenix by Bosnalijek, Pantecta by Novartis, Pantoloc or Somac by Nycomed, Tecta by Nycomed in Canada, Protium in the UK, Inipomp by Sanofi-Aventis or Eupantol by Nycomed in France, Pantozol by Nycomed in Germany, Pantodac, Perizole by Obsurge Biotech and Pansped in India, Zurcazol in Greece, Protonex by Abdi İbrahim in Turkey, Pantup in Ireland, Pantomed in Belgium, TopZole in South Africa, and as UXL-D in India and Pantid in Bangladesh. The generic pantoprazole sodium is available in the U.S. from Kremers Urban. It is available by prescription in delayed-release tablets. It is also available for intravenous use.
On 24 December 2007, Teva Pharmaceutical released an AB-rated generic alternative to Protonix.[3] This was followed by generic equivalents from Sun Pharma and Kudco Pharma. Wyeth sued all three for patent infringement and launched its own generic version of Protonix with Nycomed.[4][5]
On October 18, 2010 the U.S. Food and Drug Administration (FDA) accepted the filing of an ANDA for a delayed release generic version of Protonix by Canadian company IntelliPharmaCeutics.[6] The combination of domperidone 10 mg and pantoprazole 40 mg is available in India under the brand name Pantazone, Pantop-D, Rantop-D. In Bangladesh Apex Pharma Limited also marketed pantoprazole under the brand name Pantrol.
References
- ^ Meyer, U A (1996). "Metabolic interactions of the proton-pump inhibitors lansoprazole, omeprazole and pantoprazole with other drugs". European journal of gastroenterology & hepatology 8 (Suppl 1): S21–25. doi:10.1097/00042737-199610001-00005.
- ^ Steinijans, V. W.; Huber, R.; Hartmann, M.; Zech, K.; Bliesath, H.; Wurst, W.; Radtke, H. W. (1996). "Lack of pantoprazole drug interactions in man: An updated review". International Journal of Clinical Pharmacology and Therapeutics 34 (6): 243–262. PMID 8793611.
- ^ Teva Announces Launch Of Generic Protonix Tablets
- ^ Rubenstein, Sarah (29 January 2008). "Wyeth Plans Generic Protonix; Litigation With Teva to Continue". The Wall Street Journal: p. D9. http://online.wsj.com/article/SB120165361361326959.html. Retrieved 25 October 2009.
- ^ "Nycomed and Wyeth announce launch of an own generic version of PROTONIX - lawsuit to defend patent continues". http://www.nycomed.com/en/Menu/Media/News+releases/Protonix_30_01_08.htm. Retrieved 25 October 2009.[dead link]
- ^ IntelliPharmaCeutics Press Release [1]
External links
Drugs for acid related disorders: Drugs for peptic ulcer and GERD/GORD (A02B) H2 antagonists ("-tidine") Cimetidine • Famotidine • Lafutidine • Loxtidine • Niperotidine • Nizatidine • Ranitidine • RoxatidineProstaglandins (E)/analogues ("-prost-") Proton-pump inhibitors ("-prazole") Dexlansoprazole • Esomeprazole • Lansoprazole • Omeprazole • Pantoprazole • Rabeprazole • TenatoprazoleOther Acetoxolone • Alginic acid • Carbenoxolone • Cetraxate • Gefarnate • Pirenzepine • Proglumide • Sucralfate • Sulglicotide • Teprenone • Troxipide • Zolimidine • Rebamipide •Categories:- Proton pump inhibitors
- Benzimidazoles
- Sulfoxides
- Wyeth
- Phenol ethers
- Organofluorides
- Pyridines
Wikimedia Foundation. 2010.
