- Diphenyl oxalate
-
Diphenyl oxalate 
 diphenyl oxalateOther namesdiphenylethandioate, oxalic acid diphenyl ester, cyalume, DPO
diphenyl oxalateOther namesdiphenylethandioate, oxalic acid diphenyl ester, cyalume, DPOIdentifiers CAS number 3155-16-6 PubChem 18475 Jmol-3D images Image 1 - O=C(Oc1ccccc1)C(=O)Oc2ccccc2
Properties Molecular formula C14H10O4 Molar mass 242.227 g/mol Appearance solid Melting point 136 °C, 409 K, 277 °F
 (verify) (what is:
(verify) (what is:  /
/ ?)
?)
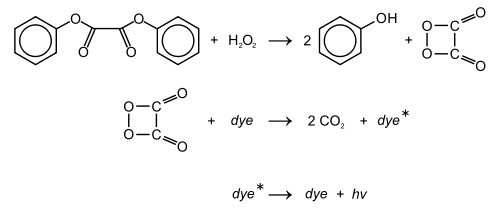
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Diphenyl oxalate (trademark name Cyalume) is a solid ester whose oxidation products are responsible for the chemiluminescence in a glowstick. It can be synthesized by fully esterifying phenol with oxalic acid. The reaction with hydrogen peroxide that diphenyl oxalate undergoes to produce a photon of light is shown below:
The reaction rate is pH dependent, and slightly alkaline conditions achieved by adding a weak base, e.g., sodium salicylate, will produce brighter light. The 2,4,6-trichlorophenol ester of oxalic acid is a solid and thus easier to handle. Furthermore, since trichlorophenolate is the better leaving group, the reaction will proceed faster, again producing brighter light, as compared to the phenol ester.
The following colors can be produced by using different dyes:
Color Compound Blue 9,10-Diphenylanthracene Green 9,10-Bis(phenylethynyl)anthracene Yellow-green Tetracene Yellow 1-Chloro-9,10-bis(phenylethynyl)anthracene Orange 5,12-Bis(phenylethynyl)naphthacene, Rubrene, Rhodamine 6G Red Rhodamine B References
The Ph balance is most important as the effects differ.Ref http://www.mpsafety.co.uk
Categories:- Oxalates
Wikimedia Foundation. 2010.