- Oxalic acid
-
Oxalic acid 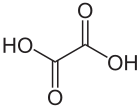
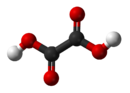
 ethanedioic acidOther namesoxalic acid
ethanedioic acidOther namesoxalic acidIdentifiers CAS number 144-62-7 
PubChem 971 ChemSpider 946 
UNII 9E7R5L6H31 
EC number 205-634-3 UN number 3261 DrugBank DB03902 KEGG C00209 
MeSH Oxalic+acid ChEBI CHEBI:16995 
ChEMBL CHEMBL146755 
RTECS number RO2450000 ATCvet code QP53 Beilstein Reference 385686 Gmelin Reference 2208 3DMet B00059 Jmol-3D images Image 1 - C(=O)(C(=O)O)O
Properties Molecular formula C2H2O4 Molar mass 90.03 g mol−1 Exact mass 89.995308552 g mol-1 Appearance White crystals Density 1.90 g cm-3 Melting point 189-191 °C, 462-464 K, 372-376 °F
Solubility in water 90 g dm-3 (at 20 °C) Acidity (pKa) 1.25, 4.14[1] Hazards MSDS External MSDS NFPA 704 Flash point 166 °C Related compounds Related compounds oxalyl chloride
disodium oxalate
calcium oxalate
phenyl oxalate ester (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Oxalic acid is an organic compound with the formula H2C2O4. This colourless solid is a dicarboxylic acid. In terms of acid strength, it is about 3,000 times stronger than acetic acid. Oxalic acid is a reducing agent and its conjugate base, known as oxalate (C2O42−), is a chelating agent for metal cations. Typically oxalic acid occurs as the dihydrate with the formula H2C2O4·2H2O.
Contents
Preparation
Oxalic acid is mainly manufactured by the oxidation of carbohydrates or glucose using nitric acid or air in the presence of vanadium pentoxide. A variety of precursors can be used including glycolic acid and ethylene glycol.[2] A newer method entails oxidative carbonylation of alcohols to give the diesters of oxalic acid:
- 4 ROH + 4 CO + O2 → 2 (CO2R)2 + 2 H2O
These diesters are subsequently hydrolyzed to oxalic acid. Approximately 120,000 metric tons are produced annually.[3]
Laboratory methods
Although it can be readily purchased, oxalic acid can be prepared in the laboratory by oxidizing sucrose using nitric acid in the presence of a small amount of vanadium pentoxide as a catalyst.[4]
The hydrated solid can be dehydrated with heat or by azeotropic distillation.[5]
Of historical interest, Wöhler prepared oxalic acid by hydrolysis of cyanogen in 1824. This experiment may represent the first synthesis of a natural product.[3]
Structure
Anhydrous oxalic acid exists as two polymorphs; in one the hydrogen-bonding results in a chain-like structure whereas the hydrogen bonding pattern in the other form defines a sheet-like structure.[6] Because the anhydrous material is both acidic and hygroscopic (water seeking), it is used in esterifications.
Reactions
Oxalic acid is a relatively strong acid, despite being a carboxylic acid:
- C2O4H2 → C2O4H− + H+; pKa = 1.38
- C2O4H− → C2O42− + H+; pKa = 4.28
Oxalic acid undergoes many of the reactions characteristic of other carboxylic acids. It forms esters such as dimethyl oxalate (m.p. 52.5–53.5 °C).[7] It forms an acid chloride called oxalyl chloride.
Oxalate, the conjugate base of oxalic acid, is an excellent ligand for metal ions, e.g. the drug oxaliplatin.
Oxalic acid and oxalates can be oxidized by permanganate in an autocatalytic reaction.[8]
Occurrence in nature
Oxalic acid and oxalates are present in many plants, including black tea, and occur naturally in animals. Calcium oxalate is the most common component of kidney stones. Early investigators isolated oxalic acid from wood-sorrel (Oxalis). Its presence makes it dangerous to eat unripe carambola or monstera fruits. Members of the spinach family are high in oxalates, as is sorrel, and a, "steady diet of raw leaves," is not recommended.[9] Rhubarb leaves contain about 0.5% oxalic acid and Jack-in-the-Pulpit (Arisaema triphyllum) contains calcium oxalate crystals. Bacteria produce oxalates from oxidation of carbohydrates.[3]
Oxidized bitumen or bitumen exposed to gamma-rays also contains oxalic acid amongst its degradation products. Oxalic acid may increase the leaching of radionuclides conditioned in bitumen for radioactive waste disposal.
Applications
Oxalic acid's main applications include cleaning or bleaching, especially for the removal of rust (iron complexing agent), e.g. Bar Keepers Friend is an example of a household cleaner containing oxalic acid. About 25% of produced oxalic acid is used as a mordant in dyeing processes. It is used in bleaches, especially for pulpwood. It is also used in baking powder.[3]
Extractive metallurgy
Oxalic acid is also an important reagent in lanthanide chemistry. Hydrated lanthanide oxalates form readily in strongly acidic solutions in a densely crystalline easily filtered form, largely free of contamination by non-lanthanide elements. Lanthanide oxalates figure importantly in commercial processing of lanthanides, and are used to recover lanthanides from solution after separation. After thermal decomposition and oxalate combustion, lanthanide oxalates convert to the oxides, which are the most common form in which the lanthanides are marketed.
Miscellaneous uses
Oxalic acid is used in the restoration of old wood. Its reducing properties are used in platinotype, the early photographic platinum/palladium printing process. Oxalic acid is also used for cleaning 'grubbyness' from dirty leather to get back to the flesh of the leather, before reintroducing preservatives.
Vaporized oxalic acid, or a 3.2% solution of oxalic acid in sugar syrup, is used by some beekeepers as a miticide against the parasitic varroa mite.
Oxalic acid is rubbed onto completed marble sculptures to seal the surface and introduce a shine.
Toxicity and safety
In humans, oxalic acid has an oral LDLo (lowest published lethal dose) of 600 mg/kg (human).[10]
The main toxicity of oxalic acid is due to the precipitation of calcium oxalate in the kidneys when urine becomes supersaturated with respect to this salt. Oxalic acid is also a metabolism product of the degradation of ethylene glycol if accidentally ingested and, as such, directly represents a danger for the kidneys in case of glycol poisoning.
References
- ^ Bjerrum, J., et al. Stability Constants, Chemical Society, London, 1958.
- ^ http://www.freepatentsonline.com/3678107.html Process for the production of oxalic acid
- ^ a b c d Wilhelm Riemenschneider, Minoru Tanifuji "Oxalic acid" in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi: 10.1002/14356007.a18_247.
- ^ Practical Organic Chemistry by Julius B. Cohen, 1930 ed. preparation #42
- ^ Clarke H. T.;. Davis, A. W. (1941), "Oxalic acid (anhydrous)", Org. Synth.: 421, http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV1P0421; Coll. Vol. 1
- ^ Wells, A.F. (1984) Structural inorganic chemistry, Oxford: Clarendon Press. ISBN 0-19-855370-6.
- ^ Bowden, E. (1943), "Methyl oxalate", Org. Synth.: 414, http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV2P0414; Coll. Vol. 2
- ^ Kovacs K.A., Grof P., Burai L., Riedel M. (2004). "Revising the mechanism of the permanganate/oxalate reaction". J. Phys. Chem. A 108 (50): 11026–11031. doi:10.1021/jp047061u.
- ^ Rombauer, Rombauer Becker, and Becker (1931/1997). Joy of Cooking, p.415. ISBN 0-684-81870-1.
- ^ Safety Officer in Physical Chemistry (August 13, 2005). "Safety (MSDS) data for oxalic acid dihydrate". Oxford University. http://msds.chem.ox.ac.uk/OX/oxalic_acid_dihydrate.html. Retrieved December 30, 2009.
External links
- International Chemical Safety Card 0529
- "Oxalic acid". ChemicalLand21.com. http://www.chemicalland21.com/arokorhi/industrialchem/organic/OXALIC%20ACID.htm.
- Table: Oxalic acid content of selected vegetables (USDA)
- Alternative link: Table: Oxalic Acid Content of Selected Vegetables (USDA)
- About rhubarb poisoning (The Rhubarb Compendium)
- Low-Oxalate Diet (PDF)[dead link]
- Oxalosis & Hyperoxaluria Foundation (OHF) The Oxalate Content of Food 2008 (PDF)
- Oxalosis & Hyperoxaluria Foundation (OHF) Diet Information
- Calculator: Water and solute activities in aqueous oxalic acid
Categories:- Oxalates
- Dicarboxylic acids
- Household chemicals
Wikimedia Foundation. 2010.

