- MMDA (drug)
-
MMDA (drug) 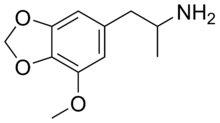
Clinical data Pregnancy cat. ? Legal status Schedule I (US) Routes Oral, Insufflation, Rectal Identifiers CAS number 13674-05-0 ATC code None PubChem CID 26175 ChemSpider 24386 Chemical data Formula C11H15NO3 Mol. mass 209.24 g/mol SMILES eMolecules & PubChem 3-Methoxy-4,5-methylenedioxyamphetamine (MMDA; 3-Methoxy-MDA) is a psychedelic and entactogen drug of the amphetamine class. It is an analogue of lophophine, MDA, and MDMA, and also bears resemblance to the essential oil myristicin found in nutmeg.
MMDA was described by Alexander Shulgin in his book PiHKAL. Shulgin lists the dosage range of MMDA as 100–250 mg. The first symptoms appear within 30–60 minutes following oral administration. MMDA produces euphoria and loving warmth, and attenuates feelings such as anxiety and loneliness. MMDA also produces eyes-closed visuals, a state of drowsiness, muscle relaxation, and time dilation. Side effects include moderate mydriasis, dizziness, sensations of heat or cold, and trembling. The imagery is generally realistic, and often related to everyday perception of people, landscapes, or objects. The effects of MMDA usually reach a peak after the first hour following the initial symptoms, and begin to wane during the second hour, and usually completely disappear by the end of the fifth hour.
Contents
Psychotherapeutic actions
In his 1973 book, "The Healing Journey", Claudio Naranjo explored the psychotherapeutic potential of MMDA. Like MDA, he found that MMDA facilitates communication and suggested it has potential applications in psychotherapy. Worldwide as of 2005[update], MMDA has not been approved for any human applications.
Pharmacology
MMDA has been shown to act as a non-neurotoxic serotonin releasing agent with no effects on dopamine release and probably norepinephrine release as well,[1] and as a 5-HT2A receptor agonist.[2] The latter property is responsible for its psychedelic effects, whereas the former mediates its mood-lifting and empathogenic effects.
Legality
MMDA is classified as a Schedule 1 substance in the United States, and is similarly controlled in other parts of the world. Internationally, MMDA is a Schedule I drug under the Convention on Psychotropic Substances[1].
See also
- Lophophine
- MMDA-2
- 5-Methyl-MDA
- MDA
- MDMA
References
- ^ McKenna DJ, Guan XM, Shulgin AT (March 1991). "3,4-Methylenedioxyamphetamine (MDA) analogues exhibit differential effects on synaptosomal release of 3H-dopamine and 3H-5-hydroxytryptamine". Pharmacology, Biochemistry, and Behavior 38 (3): 505–12. doi:10.1016/0091-3057(91)90005-M. PMID 1829838. http://linkinghub.elsevier.com/retrieve/pii/0091-3057(91)90005-M.
- ^ Zhang Z, An L, Hu W, Xiang Y (April 2007). "3D-QSAR study of hallucinogenic phenylalkylamines by using CoMFA approach". Journal of Computer-aided Molecular Design 21 (4): 145–53. doi:10.1007/s10822-006-9090-y. PMID 17203365.
External links
- PiHKAL entry for MMDA
- PiHKAL • info entry for MMDA
- MMDA: A New Psychotomimetic Agent
- Animal Pharmacology and Human Psychopharmacology of MMDA
- A Pharmacological Comparison of MMDA and LSD In The Dog
Entactogens Aminoindanes 5-IAI • ETAI • TAIAminotetralins 6-CATPhenethylamines
(and amphetamines,
cathinones, etc)4-CAB • 4-FA • 4-FMA • 4-MTA • 4-FPP • 5-APDB • 6-APDB • Ariadne • BDB • Brephedrone • Eutylone • Flephedrone • IAP • IMP • Metaescaline • Mephedrone • Methedrone • MMA • NAP • Norfenfluramine • Pentylone • PMA • PMEA • PMMA • TAPMDxx 2-Methyl-MDA • 5-Methyl-MDA • 6-Methyl-MDA • bk-MBDB (butylone) • bk-MDEA (ethylone) • bk-MDMA (methylone) • DMMDA • DMMDA-2 • EBDB • EBDP • EDMA • MDAI • MDMAI • MMAI • MDAT • MDMAT • MDA • MDDM • MDEA • MDIP • MDMA • MDMOH • MDMP • MDMPEA • MDOH • MDPEA • MDPH • MDPR • MMDPEA (Lophophine) • MBDB • MBDP • MMDA • MMDA-2 • MMDMATryptamines 4-Methyl-αET • αETSerotonergics 5-HT1 receptor ligands Agonists: Azapirones: Alnespirone • Binospirone • Buspirone • Enilospirone • Eptapirone • Gepirone • Ipsapirone • Perospirone • Revospirone • Tandospirone • Tiospirone • Umespirone • Zalospirone; Antidepressants: Etoperidone • Nefazodone • Trazodone • Vortioxetine; Antipsychotics: Aripiprazole • Asenapine • Clozapine • Quetiapine • Ziprasidone; Ergolines: Dihydroergotamine • Ergotamine • Lisuride • Methysergide • LSD; Tryptamines: 5-CT • 5-MeO-DMT • 5-MT • Bufotenin • DMT • Indorenate • Psilocin • Psilocybin; Others: 8-OH-DPAT • Adatanserin • Befiradol • BMY-14802 • Cannabidiol • Dimemebfe • Ebalzotan • Eltoprazine • F-11,461 • F-12,826 • F-13,714 • F-14,679 • F-15,063 • F-15,599 • Flesinoxan • Flibanserin • Lesopitron • LY-293,284 • LY-301,317 • MKC-242 • NBUMP • Osemozotan • Oxaflozane • Pardoprunox • Piclozotan • Rauwolscine • Repinotan • Roxindole • RU-24,969 • S 14,506 • S-14,671 • S-15,535 • Sarizotan • SSR-181,507 • Sunepitron • U-92,016-A • Urapidil • Vilazodone • Xaliproden • Yohimbine
Antagonists: Antipsychotics: Iloperidone • Risperidone • Sertindole; Beta blockers: Alprenolol • Cyanopindolol • Iodocyanopindolol • Oxprenolol • Pindobind • Pindolol • Propranolol • Tertatolol; Others: AV965 • BMY-7,378 • CSP-2503 • Dotarizine • Flopropione • GR-46611 • Isamoltane • Lecozotan • Mefway • Metitepine/Methiothepin • MPPF • NAN-190 • PRX-00023 • Robalzotan • S-15535 • SB-649,915 • SDZ 216-525 • Spiperone • Spiramide • Spiroxatrine • UH-301 • WAY-100,135 • WAY-100,635 • XylamidineAgonists: Lysergamides: Dihydroergotamine • Ergotamine • Methysergide; Piperazines: Eltoprazine • TFMPP; Triptans: Avitriptan • Eletriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-MT; Others: CGS-12066A • CP-93,129 • CP-94,253 • CP-135,807 • RU-24,969
Antagonists: Lysergamides: Metergoline; Others: AR-A000002 • Elzasonan • GR-127,935 • Isamoltane • Metitepine/Methiothepin • SB-216,641 • SB-224,289 • SB-236,057 • YohimbineAgonists: Lysergamides: Dihydroergotamine • Methysergide; Triptans: Almotriptan • Avitriptan • Eletriptan • Frovatriptan • Naratriptan • Rizatriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-Ethyl-DMT • 5-MT • 5-(Nonyloxy)tryptamine; Others: CP-135,807 • CP-286,601 • GR-46611 • L-694,247 • L-772,405 • PNU-109,291 • PNU-142,633
Antagonists: Lysergamides: Metergoline; Others: Alniditan • BRL-15,572 • Elzasonan • GR-127,935 • Ketanserin • LY-310,762 • LY-367,642 • LY-456,219 • LY-456,220 • Metitepine/Methiothepin • Ritanserin • Yohimbine • ZiprasidoneAgonists: Lysergamides: Methysergide; Triptans: Eletriptan; Tryptamines: BRL-54443 • Tryptamine
Antagonists: Metitepine/MethiothepinAgonists: Triptans: Eletriptan • Naratriptan • Sumatriptan; Tryptamines: 5-MT; Others: BRL-54443 • Lasmiditan • LY-334,370
Antagonists: Metitepine/Methiothepin5-HT2 receptor ligands Agonists: Lysergamides: ALD-52 • Ergometrine • Lisuride • LA-SS-Az • LSD • LSD-Pip • Lysergic acid 2-butyl amide • Lysergic acid 3-pentyl amide • Methysergide; Phenethylamines: 25I-NBF • 25I-NBMD • 25I-NBOH • 25I-NBOMe • 2C-B • 2C-B-FLY • 2CB-Ind • 2C-C-NBOMe • 2C-E • 2C-I • 2C-TFM-NBOMe • 2C-T-2 • 2C-T-7 • 2C-T-21 • 2CBCB-NBOMe • 2CBFly-NBOMe • Bromo-DragonFLY • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline • TCB-2 • TFMFly; Piperazines: BZP • Quipazine • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: AL-34662 • AL-37350A • Dimemebfe • Medifoxamine • Oxaflozane • PNU-22394 • RH-34
Antagonists: Atypical antipsychotics: Amperozide • Aripiprazole • Carpipramine • Clocapramine • Clozapine • Gevotroline • Iloperidone • Melperone • Mosapramine • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Loxapine • Pipamperone; Antidepressants: Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Mianserin • Mirtazapine • Nefazodone • Teniloxazine • Trazodone; Others: 5-I-R91150 • AC-90179 • Adatanserin • Altanserin • AMDA • APD-215 • Blonanserin • Cinanserin • CSP-2503 • Cyproheptadine • Deramciclane • Dotarizine • Eplivanserin • Esmirtazapine • Fananserin • Flibanserin • Ketanserin • KML-010 • Lubazodone • Mepiprazole • Metitepine/Methiothepin • Nantenine • Pimavanserin • Pizotifen • Pruvanserin • Rauwolscine • Ritanserin • S-14,671 • Sarpogrelate • Setoperone • Spiperone • Spiramide • SR-46349B • Volinanserin • Xylamidine • YohimbineAgonists: Oxazolines: 4-Methylaminorex • Aminorex; Phenethylamines: Chlorphentermine • Cloforex • DOB • DOC • DOI • DOM • Fenfluramine • MDA • MDMA • Norfenfluramine; Tryptamines: 5-CT • 5-MT • α-Methyl-5-HT; Others: BW-723C86 • Cabergoline • mCPP • Pergolide • PNU-22394 • Ro60-0175
Antagonists: Agomelatine • Asenapine • EGIS-7625 • Ketanserin • Lisuride • LY-272,015 • Metitepine/Methiothepin • PRX-08066 • Rauwolscine • Ritanserin • RS-127,445 • Sarpogrelate • SB-200,646 • SB-204,741 • SB-206,553 • SB-215,505 • SB-221,284 • SB-228,357 • SDZ SER-082 • Tegaserod • YohimbineAgonists: Phenethylamines: 2C-B • 2C-E • 2C-I • 2C-T-2 • 2C-T-7 • 2C-T-21 • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline; Piperazines: Aripiprazole • mCPP • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: A-372,159 • AL-38022A • CP-809,101 • Dimemebfe • Lorcaserin• Medifoxamine • MK-212 • Org 12,962 • ORG-37,684 • Oxaflozane • PNU-22394 • Ro60-0175 • Ro60-0213 • Vabicaserin • WAY-629 • WAY-161,503 • YM-348
Antagonists: Atypical antipsychotics: Clozapine • Iloperidone • Melperone • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine • Pipamperone; Antidepressants: Agomelatine • Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Fluoxetine • Mianserin • Mirtazapine • Nefazodone • Nortriptyline • Tedatioxetine • Trazodone; Others: Adatanserin • Cinanserin • Cyproheptadine • Deramciclane • Dotarizine • Eltoprazine • Esmirtazapine • FR-260,010 • Ketanserin • Ketotifen • Latrepirdine • Metitepine/Methiothepin • Methysergide • Pizotifen • Ritanserin • RS-102,221 • S-14,671 • SB-200,646 • SB-206,553 • SB-221,284 • SB-228,357 • SB-242,084 • SB-243,213 • SDZ SER-082 • Xylamidine5-HT3, 5-HT4, 5-HT5, 5-HT6, 5-HT7 ligands Agonists: Piperazines: BZP • Quipazine; Tryptamines: 2-Methyl-5-HT • 5-CT; Others: Chlorophenylbiguanide • Butanol • Ethanol • Halothane • Isoflurane • RS-56812 • SR-57,227 • SR-57,227-A • Toluene • Trichloroethane • Trichloroethanol • Trichloroethylene • YM-31636
Antagonists: Antiemetics: AS-8112 • Alosetron • Azasetron • Batanopride • Bemesetron • Cilansetron • Dazopride • Dolasetron • Granisetron • Lerisetron • Ondansetron • Palonosetron • Ramosetron • Renzapride • Tropisetron • Zacopride • Zatosetron; Atypical antipsychotics: Clozapine • Olanzapine • Quetiapine; Tetracyclic antidepressants: Amoxapine • Mianserin • Mirtazapine; Others: CSP-2503 • ICS-205,930 • MDL-72,222 • Memantine • Nitrous Oxide • Ricasetron • Sevoflurane • Tedatioxetine • Thujone • Vortioxetine • XenonAgonists: Gastroprokinetic Agents: Cinitapride • Cisapride • Dazopride • Metoclopramide • Mosapride • Prucalopride • Renzapride • Tegaserod • Velusetrag • Zacopride; Others: 5-MT • BIMU8 • CJ-033,466 • PRX-03140 • RS-67333 • RS-67506 • SL65.0155 • Antagonists: GR-113,808 • GR-125,487 • L-Lysine • Piboserod • RS-39604 • RS-67532 • SB-203,186 • SB-204,070Agonists: Lysergamides: Ergotamine • LSD; Tryptamines: 5-CT; Others: Valerenic Acid
Antagonists: Asenapine • Latrepirdine • Metitepine/Methiothepin • Ritanserin • SB-699,551
* Note that the 5-HT5B receptor is not functional in humans.Agonists: Lysergamides: Dihydroergotamine • Ergotamine • Lisuride • LSD • Mesulergine • Metergoline • Methysergide; Tryptamines: 2-Methyl-5-HT • 5-BT • 5-CT • 5-MT • Bufotenin • E-6801 • E-6837 • EMD-386,088 • EMDT • LY-586,713 • Tryptamine; Others: WAY-181,187 • WAY-208,466
Antagonists: Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Doxepin • Mianserin • Nortriptyline; Atypical antipsychotics: Aripiprazole • Asenapine • Clozapine • Fluperlapine • Iloperidone • Olanzapine • Tiospirone; Typical antipsychotics: Chlorpromazine • Loxapine; Others: BGC20-760 • BVT-5182 • BVT-74316 • Cerlapirdine • EGIS-12,233 • GW-742,457 • Ketanserin • Latrepirdine • Lu AE58054 • Metitepine/Methiothepin • MS-245 • PRX-07034 • Ritanserin • Ro04-6790 • Ro 63-0563 • SB-258,585 • SB-271,046 • SB-357,134 • SB-399,885 • SB-742,457Agonists: Lysergamides: LSD; Tryptamines: 5-CT • 5-MT • Bufotenin; Others: 8-OH-DPAT • AS-19 • Bifeprunox • E-55888 • LP-12 • LP-44 • RU-24,969 • Sarizotan
Antagonists: Lysergamides: 2-Bromo-LSD • Bromocriptine • Dihydroergotamine • Ergotamine • Mesulergine • Metergoline • Methysergide; Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Imipramine • Maprotiline • Mianserin; Atypical antipsychotics: Amisulpride • Aripiprazole • Clozapine • Olanzapine • Risperidone • Sertindole • Tiospirone • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine; Others: Butaclamol • EGIS-12,233 • Ketanserin • LY-215,840 • Metitepine/Methiothepin • Pimozide • Ritanserin • SB-258,719 • SB-258,741 • SB-269,970 • SB-656,104 • SB-656,104-A • SB-691,673 • SLV-313 • SLV-314 • Spiperone • SSR-181,507Reuptake inhibitors Selective serotonin reuptake inhibitors (SSRIs): Alaproclate • Citalopram • Dapoxetine • Desmethylcitalopram • Desmethylsertraline • Escitalopram • Femoxetine • Fluoxetine • Fluvoxamine • Indalpine • Ifoxetine • Litoxetine • Lubazodone • Panuramine • Paroxetine • Pirandamine • RTI-353 • Seproxetine • Sertraline • Tedatioxetine • Vilazodone • Vortioxetine • Zimelidine; Serotonin-norepinephrine reuptake inhibitors (SNRIs): Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors (SNDRIs): Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • NS-2359 • SEP-225289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants (TCAs): Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • Nortriptyline • Pipofezine • Protriptyline • Trimipramine; Tetracyclic antidepressants (TeCAs): Amoxapine; Piperazines: Nefazodone • Trazodone; Antihistamines: Brompheniramine • Chlorphenamine • Diphenhydramine • Mepyramine/Pyrilamine • Pheniramine • Tripelennamine; Opioids: Pethidine • Methadone • Propoxyphene; Others: Cocaine • CP-39,332 • Cyclobenzaprine • Dextromethorphan • Dextrorphan • EXP-561 • Fezolamine • Mesembrine • Nefopam • PIM-35 • Pridefine • Roxindole • SB-649,915 • ZiprasidoneReleasing agents Aminoindanes: 5-IAI • AMMI • ETAI • MDAI • MDMAI • MMAI • TAI; Aminotetralins: 6-CAT • 8-OH-DPAT • MDAT • MDMAT; Oxazolines: 4-Methylaminorex • Aminorex • Clominorex • Fluminorex; Phenethylamines (also Amphetamines, Cathinones, Phentermines, etc): 2-Methyl-MDA • 4-CAB • 4-FA • 4-FMA • 4-HA • 4-MTA • 5-APDB • 5-Methyl-MDA • 6-APDB • 6-Methyl-MDA • AEMMA • Amiflamine • BDB • BOH • Brephedrone • Butylone • Chlorphentermine • Cloforex • Amfepramone • Metamfepramone • DFMDA • DMA • DMMA • EBDB • EDMA • Ethylone • Etolorex • Fenfluramine (Dexfenfluramine) • Flephedrone • IAP • IMP • Lophophine • MBDB • MDA • MDEA • MDHMA • MDMA • MDMPEA • MDOH • MDPEA • Mephedrone • Methedrone • Methylone • MMA • MMDA • MMDMA • MMMA • NAP • Norfenfluramine • 4-TFMA • pBA • pCA • pIA • PMA • PMEA • PMMA • TAP; Piperazines: 2C-B-BZP • 2-BZP • 3-MeOPP • BZP • DCPP • MBZP • mCPP • MDBZP • MeOPP • Mepiprazole • pCPP • pFPP • pTFMPP • TFMPP; Tryptamines: 4-Methyl-αET • 4-Methyl-αMT • 5-CT • 5-MeO-αET • 5-MeO-αMT • 5-MT • αET • αMT • DMT • Tryptamine (itself); Others: Indeloxazine • Tramadol • ViqualineEnzyme inhibitors AGN-2979 • FenclonineNonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A Selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • TyrimaOthers Ferrous iron (Fe2+) • Magnesium (Mg2+) • Tetrahydrobiopterin • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal phosphate) • Vitamin B9 (Folic Acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersPhenethylamines Phenethylamines Psychedelics: 2C-B • 2C-B-FLY • 2C-C • 2C-D • 2C-E • 2C-F • 2C-G • 2C-I • 2C-N • 2C-P • 2C-SE • 2C-T • 2C-T-2 • 2C-T-4 • 2C-T-7 • 2C-T-8 • 2C-T-9 • 2C-T-13 • 2C-T-15 • 2C-T-17 • 2C-T-21 • 2C-TFM • 2C-YN • Allylescaline • DESOXY • Escaline • Isoproscaline • Jimscaline • Macromerine • MEPEA • Mescaline • Metaescaline • Methallylescaline • Proscaline • Psi-2C-T-4 • TCB-2
Stimulants: 2-OH-PEA • β-Me-PEA • Hordenine • N-Me-PEA • Phenethylamine (PEA)
Entactogens: Lophophine • MDPEA • MDMPEA
Others: BOH • DMPEAAmphetamines
PhenylisopropylaminesPsychedelics: 3C-BZ • 3C-E • 3C-P • Aleph • Beatrice • Bromo-DragonFLY • D-Deprenyl • DMA • DMCPA • DMMDA • DOB • DOC • DOEF • DOET • DOI • DOM • DON • DOPR • DOTFM • Ganesha • MMDA • MMDA-2 • Psi-DOM • TMA • TeMA
Stimulants: 4-MA • 4-MMA • 4-MTA • 5-IT • Alfetamine • Amfecloral • Amfepentorex • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • Benfluorex • Benzphetamine • Cathine • Clobenzorex • Dimethylamphetamine • Ephedrine (EPH) • Ethylamphetamine • Fencamfamine • Fencamine • Fenethylline • Fenfluramine (Dexfenfluramine) • Fenproporex • Fludorex • Furfenorex • Isopropylamphetamine • Lefetamine • Mefenorex • Methamphetamine (Dextromethamphetamine, Levomethamphetamine) • Methoxyphenamine • MMA • Norfenfluramine • Oxilofrine • Ortetamine • PBA • PCA • Phenpromethamine • PFA • PFMA • PIA • PMA • PMEA • PMMA • Phenylpropanolamine (PPA) • Prenylamine • Propylamphetamine • Pseudoephedrine (PSE) • Sibutramine • Tiflorex (Flutiorex) • Tranylcypromine • Xylopropamine • Zylofuramine
Entactogens: 5-APDB • 6-APB • 6-APDB • EDA • IAP • MDA • MDEA • MDHMA (FLEA) • MDMA ("Ecstasy") • MDOH • MMDMA • NAP • TAP
Others: Amiflamine • DFMDA • D-Deprenyl • L-Deprenyl (Selegiline)Phentermines Stimulants: Chlorphentermine • Cloforex • Clortermine • Etolorex • Mephentermine • Pentorex (Phenpentermine) • Phentermine
Entactogens: MDPH • MDMPHCathinones Stimulants: Amfepramone • Brephedrone • Buphedrone • Bupropion (Amfebutamone) • Cathinone (Propion) • Dimethylcathinone (Dimethylpropion, Metamfepramone) • Ethcathinone (Ethylpropion) • Flephedrone • Methcathinone (Methylpropion) • Mephedrone • Methedrone
Entactogens: Ethylone • MethylonePhenylisobutylamines Phenylalkylpyrrolidines Stimulants: α-PBP • α-PPP • α-PVP • MDPBP • MDPPP • MDPV • MOPPP • MPBP • MPHP • MPPP • Naphyrone • PEP • Prolintane • PyrovaleroneCatecholamines
(and relatives..)6-FNE • 6-OHDA • α-Me-DA • α-Me-TRA • Adrenochrome • Ciladopa • D-DOPA (Dextrodopa) • Dopamine • Epinephrine (Adrenaline) • Epinine • Fenclonine • Ibopamine • L-DOPA (Levodopa) • L-DOPS (Droxidopa) • L-Phenylalanine • L-Tyrosine • meta-Octopamine • meta-Tyramine • Metanephrine • Metirosine • Methyldopa • Nordefrin (Levonordefrin) • Norepinephrine (Noradrenaline) • Normetanephrine • para-Octopamine • para-TyramineMiscellaneous Amidephrine • Arbutamine • Cafedrine • Denopamine • Dobutamine • Dopexamine • Etafedrine • Ethylnorepinephrine • Etilefrine • Famprofazone • Gepefrine • Isoprenaline (Isoproterenol) • Isoetarine • Metaraminol • Metaterol • Methoxamine • Norfenefrine • Orciprenaline • Phenylephrine (Neosynephrine) • Phenoxybenzamine • Prenalterol • Pronethalol • Propranolol • Salbutamol (Albuterol; Levosalbutamol) • Synephrine (Oxedrine) • Theodrenaline • XamoterolDrugs from PiHKAL AEM • AL • Aleph • Aleph-2 • Aleph-4 • Aleph-6 • Aleph-7 • Ariadne • Asymbescaline • Buscaline • Beatrice • Bis-TOM • BOB • BOD • BOH • BOHD • BOM • 4-Bromo-3,5-dimethoxyamphetamine • 2-Bromo-4,5-methylenedioxyamphetamine • 2C-B • 3C-BZ • 2C-C • 2C-D • 2C-E • 3C-E • 2C-F • 2C-G • 2C-G-3 • 2C-G-4 • 2C-G-5 • 2C-G-N • 2C-H • 2C-I • 2C-N • 2C-O • 2C-O-4 • 2C-P • CPM • 2C-SE • 2C-T • 2C-T-2 • 2C-T-4 • psi-2C-T-4 • 2C-T-7 • 2C-T-8 • 2C-T-9 • 2C-T-13 • 2C-T-15 • 2C-T-17 • 2C-T-21 • 4-D • beta-D • DESOXY • 2,4-DMA • 2,5-DMA • 3,4-DMA • DMCPA • DME • DMMDA • DMMDA-2 • DMPEA • DOAM • DOB • DOBU • DOC • DOEF • DOET • DOI • DOM • psi-DOM • DON • DOPR • Escaline • EEE • EEM • EME • EMM • Ethyl-J (EBDB) • Ethyl-K • F-2 • F-22 • Flea • G-3 • G-4 • G-5 • G-N • Ganesha • HOT-2 • HOT-7 • HOT-17 • IDNNA • Isomescaline • Isoproscaline • Iris • J (BDB) • Lophophine • PMA • Mescaline • Madam-6 • Methallylescaline • MDA • MDAL • MDBU • MDBZ • MDCPM • MDDM • MDE • MDHOET • MDIP • MDMA • MDMC (EDMA) • MDMEO • MDMEOET • MDMP • MDOH • MDPEA • MDPH • MDPL • MDPR • Metaescaline • MEDA • MEE • MEM • MEPEA • Meta-DOB • Meta-DOT • Methyl-DMA • Methyl-DOB • Methyl-J (MBDB) • Methyl-K • Methyl-MA (PMMA) • Methyl-MMDA-2 • MMDA • MMDA-2 • MMDA-3a • MMDA-3b • MME • Metaproscaline • MPM • Ortho-DOT • Proscaline • Phenescaline • Phenethylamine • Propynyl • Symbescaline • 2,3,4,5-Tetramethoxyamphetamine • 3-TASB • 4-TASB • 5-TASB • Thiobuscaline • 3-TE • 4-TE • 3-TIM • 4-TIM • 5-TIM • 3-TM • 4-TM • TMA • TMA-2 • TMA-3 • TMA-4 • TMA-5 • TMA-6 • 3-TME • 4-TME • 5-TME • 2T-MMDA-3a • 4T-MMDA-2 • 2-TOET • 5-TOET • 2-TOM • 5-TOM • TOMSO • Thioproscaline • Trisescaline • 3-TSB • 4-TSB • 3-T-Trisescaline • 4-T-Trisescaline
Categories:- Amphetamines
- Alkaloids
- Entactogens and Empathogens
- Benzodioxoles
- Phenol ethers
Wikimedia Foundation. 2010.
