- Salvia divinorum
-
Salvia divinorum 
Scientific classification Kingdom: Plantae (unranked): Angiosperms (unranked): Eudicots (unranked): Asterids Order: Lamiales Family: Lamiaceae Genus: Salvia Species: S. divinorum Binomial name Salvia divinorum
Epling & Játiva[1]Salvia divinorum (also known as Diviner's Sage,[2] Ska María Pastora,[3] Seer's Sage,[4] and by its genus name Salvia) is a psychoactive plant which can induce dissociative effects and is a potent producer of "visions" and other hallucinatory experiences. Its native habitat is within cloud forest in the isolated Sierra Mazateca of Oaxaca, Mexico, where it grows in shady and moist locations.[5][6] The plant grows to over a meter high,[1] has hollow square stems, large leaves, and occasional white flowers with violet calyxes. Botanists have not determined whether Salvia divinorum is a cultigen or a hybrid; native plants reproduce vegetatively, rarely producing viable seed.[7][8]
Mazatec shamans have a long and continuous tradition of religious use of Salvia divinorum, using it to facilitate visionary states of consciousness during spiritual healing sessions.[1] Most of the plant's local common names allude to the Mazatec belief that the plant is an incarnation of the Virgin Mary, with its ritual use also invoking that relationship. Its active psychoactive constituent is a structurally unique diterpenoid called salvinorin A,[9][10] a potent κ-opioid and D2 receptor agonist.[11][12] Salvia divinorum is generally understood to be of low toxicity (high LD50)[13][14] and low addictive potential[11][15] since it is a κ-opioid agonist.
Salvia divinorum remains legal in most countries and, within the United States, is legal in the majority of states. However, some have called for its prohibition. While not currently regulated by US federal drug laws, several states have passed laws criminalizing the substance.[16] Some proposed state bills have failed to progress and have not been made into law (with motions having been voted down or otherwise dying in committee stages). There have not been many publicized prosecutions of individuals violating anti-salvia laws in the few countries and states in which it has been made illegal.[nb 1]
Contents
History
Salvia divinorum is native to the Sierra Mazateca in Oaxaca, Mexico, where it is still used by the Mazatec, primarily to facilitate shamanic visions in the context of curing or divination. S. divinorum is one of several species with hallucinogenic properties that are ritually used by Mazatec shamans. Others include certain morning glory seeds (Turbina corymbosa), psilocybin mushrooms, and various coleus species. In their rituals, the shamans use only fresh S. divinorum leaves. They see the plant as an incarnation of the Virgin Mary, and begin the ritual with an invocation to Mary, Saint Peter, the Holy Trinity, and other saints.[1] Ritual use traditionally involves being in a quiet place after ingestion of the leaf—the Maztec shamans say that "La Maria (S. divinorum) speaks with a quiet voice."[8]
It is also used remedially at lower dosages as a diuretic, and to treat ailments including diarrhea, anemia, headaches, rheumatism, and a semi-magical disease known as panzón de borrego, or a swollen belly (literally, "lamb belly").[10][15]
The history of the plant is not well known, and there has been no definitive answer to the question of its origin. Speculation includes Salvia divinorum being a wild plant native to the area; a cultigen of the Mazatecs; or a cultigen introduced by another indigenous group. Botanists have also not been able to determine whether it is a hybrid or a cultigen.[21]
Academic discovery
Salvia divinorum was first recorded in print by Jean Basset Johnson in 1939 while he was studying Mazatec shamanism.[22] He later documented its usage and reported its effects through personal testimonials.[23] It was not until the 1990s that the psychoactive mechanism was identified by a team led by Daniel Siebert.[24]
Gordon Wasson tentatively postulated that the plant could be the mythological pipiltzintzintli, the "Noble Prince" of the Aztec codices.[3][25] Wasson's speculation has been the subject of further debate amongst ethnobotanists, with some scepticism coming from Leander J. Valdés,[26] and counterpoints more supportive of Wasson's theory from Jonathan Ott.[27]
The identity of another mysterious Aztec entheogen, namely that of poyomatli, has also been suggested as being Salvia divinorum.[28] Here too there are other candidate plants, notably Cacahuaxochitl (Quararibea funebris),[29] again suggesting that there is no overall consensus.
Etymology
The genus name, Salvia, was first used by Pliny for a plant that was likely Salvia officinalis (common sage) and is derived from the Latin salvere.[30][31] The specific epithet, divinorum, was given because of the plant's traditional use in divination and healing.[5] it is often loosely translated as "diviner's sage" or "seer's sage".[4] Albert Hofmann, who collected the first plants with Wasson, objected to the new plant being given the name divinorum:
I was not very happy with the name because Salvia divinorum means "Salvia of the ghosts", whereas Salvia divinatorum, the correct name, means "Salvia of the priests", But it is now in the botanical literature under the name Salvia divinorum.[32]
In fact, the primary meaning of divinorum is "of the divine ones", although "of the soothsayers" is also a possible correct reading of it. An alternative choice of words (divinatorum, haruspicum, hariolorum...) would less ambiguously express "of the soothsayers/diviners".
There are many common names for S. divinorum, most of them relating to the plant's association with the Virgin Mary. The Mazatec believe the plant to be an incarnation of the Virgin Mary, so they take great care in handling the plant. The name "Ska Maria Pastora", often shortened to "Ska Maria" or "Ska Pastora", refers to "the leaf or herb of Mary, the Shepherdess." Other Spanish names include "hojas de Maria", "hojas de la Pastora", "hierba (yerba) Maria", and "la Maria". A plant believed to be S. divinorum was referred to as "hoja de adivinacion" (leaf of prophecy) by the Cuicatec and Mazatec.[1] S. divinorum is also known as la hembra ("the female"), when it is included by the Mazatec as part of a family of similar religious hallucinogens. The others it is connected with are Coleus pumila, called el macho ("the male"), and two forms of Coleus blumei which are called el nene ("the child") and el ahijado ("the godson").[21]
Some researchers see the lack of an indigenous Mazatec name as demonstrating a non-Mazatec origin for the plant. Others point out that the Virgin Mary is not normally viewed as a shepherdess in Christianity, and that image may hint at a pre-Hispanic Mazatec cultural reference to the plant.[21]
Recent history
Salvia divinorum has become both increasingly well-known and available in modern culture. The Internet has allowed for the growth of many businesses selling live salvia plants, dried leaves, extracts, and other preparations.
Medical experts, as well as accident and emergency rooms, have not been reporting cases that suggest particular salvia-related health concerns, and police have not been reporting it as a significant issue with regard to public order offences;[33] in any case, Salvia divinorum has attracted negative attention from the media and some lawmakers.[34]
Media stories generally raise alarms over Salvia divinorum's legal status and are sometimes headlined with generally ill-supported comparisons to LSD or other psychoactive substances. Parental concerns are raised by focusing on salvia's usage by younger teens—the emergence of YouTube videos purporting to depict its use being an area of particular concern in this respect.[citation needed] The isolated and controversial suicide of Brett Chidester received much media attention.
Salvia divinorum was the subject of the first use of YouTube within drug-behavioral research when scientists at San Diego State University rated randomly selected videos of salvia users to study observed impairment.[35][36] Their findings corroborate reports that the most profound effects of smoking salvia appear almost immediately and last about eight minutes. Effects include temporary speech and coordination loss.[37]
Botany
Salvia divinorum has large green ovate (oftentimes also dentate) leaves,[38] with a yellow undertone that reach 10 to 30 cm (4 to 12 in) long. The leaves have no hairs on either surface, and little or no petiole.[6] The plant grows to well over 1 metre (3 ft) in height,[1] on hollow square stems which tend to break or trail on the ground, with the plant rooting quite readily at the nodes and internodes.[39]
The flowers, which bloom only rarely, grow in whorls on a 30-centimetre (12 in) inflorescence, with about six flowers to each whorl. The 3-centimetre (1.2 in) flowers are white, curved and covered with hairs, and held in a small violet calyx that is covered in hairs and glands. When it does bloom in its native habitat, it does so from September to May.[39]
Early authors erred in describing the flowers as having blue corollas, based on Epling and Játiva's description. The first plant material they received was dried, so they based the flower color on an erroneous description by Hofmann and Wasson, who didn't realize that their "blue flowers, crowned with a white dome" were in fact violet calyces with unopened white corollas.[1][39][40]
Distribution and habitat
Salvia divinorum is endemic to the Sierra Mazateca in the state of Oaxaca in Mexico, growing in the primary or secondary cloud forest and tropical evergreen forest at elevations from 1,000 to 6,000 feet (300 to 1,800 m). Its most common habitat is black soil along stream banks where small trees and bushes provide an environment of low light and high humidity.[39][41]
Reproduction
Salvia divinorum produces few viable seeds even when it does flower—no seeds have ever been observed on plants in the wild. For an unknown reason, pollen fertility is also comparatively reduced. There is no active pollen tube inhibition within the style, but some event or process after the pollen tube reaches the ovary is aberrant. The likeliest explanations are inbreeding depression or hybridity.[8] All of the Mazatec populations appear to be clonal.[42] The plant's square stems break easily and tend to trail on the ground, rooting easily at the nodes and internodes.[43]
Taxonomy
Salvia divinorum was first documented in 1939, but it was many years before botanists could identify the plant due to Mazatec secrecy about the growing sites.[5] Flowers were needed for a definitive identification of the species. In 1962, the Swiss chemist Albert Hofmann, and ethnomycologist R. Gordon Wasson, traveled throughout the Sierra Mazateca researching Mazatec rituals and looking for specimens of the plant. They were unable to locate live plants. Eventually, the Mazatec provided them some flowering specimens.[22] These specimens were sent to botanists Carl Epling and Carlos D. Játiva, who described and named the plant as Salvia divinorum, after its use in divination and healing by the Mazatec.[5] By 1985, up to fifteen populations of the plant had been found.[39]
Until 2010, there were differing opinions on whether Salvia divinorum is an interspecific hybrid. The plant's partial sterility was suggestive of a hybrid origin, though no two parent species have been found with an obvious affinity to Salvia divinorum.[8][6][7] One other possibility for the plant's partial sterility is that long-term cultivation and selection have produced an inbred cultigen.[8][nb 2]
In 2010, a molecular phylogenetic approach by DNA sequencing of Salvia divinorum and a number of related species strongly suggest that the species is not a hybrid.[44] One earlier proposed parent was Salvia cyanea (a synonym for Salvia concolor), which Epling and Játiva believed to be closely allied to Salvia divinorum.[41][45][nb 3] The 2010 study demonstrated Salvia divinorum's closest relative to be Salvia venulosa—a rare and endemic Salvia that is native to Colombia, growing in shaded, wooded gullies at 1,500 to 2,000 m (4,900 to 6,600 ft) elevation. It also showed that Salvia divinorum does not belong to the Salvia section Dusenostachys, as believed earlier. The genetic study also indicated that Salvia venulosa was likely misplaced into Salvia section Tubiflorae, and that it may not be related to other Colombia Salvia species, though further tests are needed.[44]
The origin of Salvia divinorum is still a mystery, one of only three plants in the extensive Salvia genus (approximately 900 species) with unknown origins—the other two are Salvia tingitana and Salvia buchananii.[46]
Strains
There are two commonly cultivated strains which are known to be distinct. One is the strain that was collected in 1962 by ecologist and psychologist Sterling Bunnell (the Bunnell strain), colloquially mis-attributed as the Wasson-Hofmann strain. The other was collected from Huautla de Jiménez in 1991 by anthropologist Bret Blosser (the Blosser or Palatable strain).[47][48] There are other strains that are not as well documented, such as the Luna strain (possibly Bunnell) isolated from a Hawaiian patch of Salvia divinorum clones, featuring unusually serrated and rounded rather than ovate leaves.[49]
Cultivation
Propagation by cuttings
Salvia divinorum is usually propagated through vegetative reproduction. Small cuttings, between two and eight inches long, cut off of the mother plant just below a node, will usually root in plain tap water within two or three weeks.[50][51]
Flowering
Blooms occur when the day length becomes shorter than 12 hours (beginning in mid-October in some places), necessitating a shade cloth in urban environments with exposure to light pollution (HPS).[52]
Chemistry
For more details on this topic, see Salvinorin A.The known active constituent of Salvia divinorum is a trans-neoclerodane diterpenoid known as salvinorin A (chemical formula C23H28O8).[53] This compound is present in the dried plant at about 0.18%.[27]
Salvinorin A is not an alkaloid, (meaning it does not contain a basic nitrogen), unlike most known opioid receptor ligands.[54] Salvinorin A is the first documented diterpene hallucinogen.[38]
Similar to many psychoactive herbs, Salvia divinorum synthesizes and excretes its active constituent (salvinorin A) via trichomes, of the peltate-glandular morphology, located just beneath the cuticle (subcuticular) layer.[55][56]
Potency
By mass, salvinorin A "is the most potent naturally occurring hallucinogen."[57] It is active at doses as low as 200 µg.[24][53][57] Synthetic chemicals, such as LSD (active at 20–30 µg doses), can be more potent.[58] Research has shown that salvinorin A is a potent and selective κ-Opioid (kappa-Opioid) receptor agonist.[53][59] It has been reported that the effects of salvinorin A in mice are blocked by κ-Opioid receptor antagonists.[11] However, it is an even more potent D2 receptor partial agonist, and it is likely this action plays a significant role in its effects as well.[12] Salvinorin A has no actions at the 5-HT2A serotonin receptor, the principal molecular target responsible for the actions of 'classic' hallucinogens, such as mescaline and LSD, nor is it known to have affinity for any other sites to date.[11]
Salvinorin's potency should not be confused with toxicity. Rodents chronically exposed to dosages, many times greater than those to which humans are exposed, did not show signs of organ damage.[13]
Other terpenoids
Main article: Salvinorin A#Salvinorins A - F, JOther terpenoids have been isolated from Salvia divinorum, including other salvinorins and related compounds named divinatorins and salvinicins.[60] None of these compounds has shown significant (sub-micromolar) affinity at the κ-Opioid receptor, and there is no evidence that they contribute to the plant's psychoactivity.[61][62]
Other pharmaceutical action
Salvinorin A is capable of inhibiting excess intestinal motility (e.g. diarrhea), through a combination of κ-opioid and cannabinoid (mainly CB1 receptor) receptors in inflamed but not normal gut in vivo. The mechanism of action for Salvinorin A on ileal tissue has been described as 'prejunctional', as it was able to modify electrically-induced contractions, but not those of exogenous acetylcholine[63] Results from a small study by an assistant professor at the University of Iowa indicate that it may have potential as an analgesic and as a therapeutic tool for treating drug addictions.[64][65]
A pharmacologically-important aspect of the contraction-reducing (antispasmodic) properties of ingested Salvinorin A on gut tissue is that it is only pharmacologically active on inflamed and not normal tissue, thus reducing possible side-effects.[66]
Ingestion
There are a few ways to consume Salvia divinorum. In traditional Mazatec ritual, shamans use only fresh Salvia divinorum leaves. Modern methods have been developed to more effectively absorb the active principle, salvinorin A. If enough salvinorin A is absorbed, an altered state of consciousness can occur. The duration of experience varies with the method of ingestion and the amount of salvinorin A absorbed.
Traditional methods
Mazatec shamans crush the leaves to extract leaf juices from about 20 to 80 (about 50g/2 oz to 200g/7 oz.) or more fresh leaves. They usually mix these juices with water to create an infusion or 'tea' which they drink to induce visions in ritual healing ceremonies.[15]
Chewing and swallowing a large number of fresh leaves is the other Mazatec method.[67][68] Oral consumption of the leaf makes the effects come on more slowly, over a period of 10 to 20 minutes. The experience, from the onset of effects, lasts from about 30 minutes up to one and a half hours.[69]
Doses for chewing vastly exceed doses used for smoking. By calculating the concentrations per leaf ("an average concentration of 2.45 mg per gram" of leaf[70]), the average weight per leaf ("about 50 g" per 20 leaves, or 2.5g/leaf[71]), and the standard dose for chewing (about 8-28 leaves[67]), the doses can range from about 50 mg to 172 mg.
Modern methods
Salvia divinorum is becoming more widely known and used in modern culture. The National Survey on Drug Use and Health, an annual US based survey sponsored by the Substance Abuse and Mental Health Services Administration (SAMHSA), for 2006 estimated that about 1.8 million persons aged 12 or older had used Salvia divinorum in their lifetime, of which approximately 750,000 had done so in that year.[72] The following year, 2007, saw the annual figure rise from 750,000 to 1 million US users.[73]
Modern methods of ingestion include smoking or chewing the leaf, or using a tincture, as described in the following sections.
Smoking
Dry leaves can be smoked in a pipe, but most users prefer the use of a water pipe to cool the smoke.[74] The temperature required to release salvinorin from the plant material is quite high (about 240°C). A cooler flame will work, but the direct application of a more intense flame, such as that of a torch lighter, is often preferred.[74]
Some find that untreated dry leaf produces unnoticeable or only light effects.[67] Concentrated preparations or extracts which may be smoked in place of untreated leaves, have become widely available. This enhanced (or "fortified") leaf is described by a number followed by an x (e.g. 5x, 10x), the multiplicative factors being generally indicative of the relative amounts of leaf concentrate, though there is no accepted standard for these claims. Other sources may use a system of color codes to form their own standards of potency; for example, "green", "yellow", and "red."
These grades of potency may be roughly indicative of the relative concentration of the active principle, (salvinorin A), but the measure should not be taken as absolute. Overall extract potency will depend on the (naturally varying) strength of the untreated leaf used in preparing the extract, as well as the efficiency of the extraction process itself. Extracts reduce the overall amount of inhalations needed to ingest a given amount of active principle, thus facilitating more powerful experiences.[75]
If salvia is smoked, then the main effects are experienced quickly. The most intense 'peak' is reached within a minute or so and lasts for 1–5 minutes, followed by a gradual tapering off. At 5–10 minutes, less intense yet still noticeable effects typically persist, giving way to a returning sense of the everyday and familiar until back to baseline after about 15 to 20 minutes.[69]
Quid chewing
The traditional method of chewing the leaves has continued in modern use. However, salvinorin A is generally considered to be inactive when orally ingested, as salvinorin A is effectively deactivated by the gastrointestinal system.[76] Therefore, in what's understood to be a modern innovation, the 'quid' of leaves is held in the mouth as long as possible in order to facilitate absorption of the active constituents through the oral mucosa.[41] 'Quid' refers to the fact that at the end of this method the user spits out the leaves rather than swallowing them because ingesting the leaves has no known effect. Chewing consumes more of the plant than smoking, and produces a longer-lasting experience.
Using a tincture
Less commonly, some may ingest salvia in the form of a tincture. This is administered sublingually, usually with the aid of a glass dropper. It may be taken diluted with water just before use, which may slightly reduce the intensity of its effects, but can also serve to lessen or avoid a stinging sensation in the mouth caused by the presence of alcohol. Tinctures vary in potency, and the effects can range from inducing a mild meditative state to bringing about a more intense visionary one.[67]
When taken as a tincture the effects and duration are similar to other methods of oral ingestion, though they may be significantly more intense, depending on extract potency.[67]
Immediate effects
Psychedelic experiences are necessarily somewhat subjective and variations in reported effects are to be expected. Aside from individual reported experiences there has been a limited amount of published work summarising the effects. D.M. Turner's book Salvinorin—The Psychedelic Essence of Salvia Divinorum quotes Daniel Siebert's summarisation, mentioning that the effects may include:[77]
- Uncontrollable laughter
- Past memories, such as revisiting places from childhood memory
- Sensations of motion, or being pulled or twisted by forces
- Visions of membranes, films and various two-dimensional surfaces
- Merging with or becoming objects
- Overlapping realities, such as the perception of being in several locations at once
There also may be synesthetic experiences.[78] Glossolalia (speaking in tongues) has been reported by Reason.[73]
A survey of salvia users found that 38% described the effects as unique in comparison to other methods of altering consciousness. 23% said the effects were like yoga, meditation or trance.[79]
One firsthand journalistic account has been published in the UK science magazine New Scientist (note: the dose for this experience was not reported):
The salvia took me on a consciousness-expanding journey unlike any other I have ever experienced. My body felt disconnected from 'me' and objects and people appeared cartoonish, surreal and marvellous. Then, as suddenly as it had began, it was over. The visions vanished and I was back in my bedroom. I spoke to my 'sitter'—the friend who was watching over me, as recommended on the packaging—but my mouth was awkward and clumsy. When I attempted to stand my coordination was off. Within a couple of minutes, however, I was fine and clear-headed, though dripping with sweat. The whole experience had lasted less than 5 minutes.There have been few books published on the subject. One notable example is Dale Pendell's work "Phamako/Poeia—Plants Powers, Poisons, and Herbcraft", which won the 1996 Firecracker Alternative Book Award[80] and has a chapter dedicated to Salvia divinorum. It includes some experience accounts:
It's very intense, I call it a reality stutter, or a reality strobing. I think that having been a test pilot, and flying in that unforgiving environment with only two feet between our wingtips, helped to prepare me for this kind of exploration.Other users have written extensive prose and/or poetry about their experiences;[81][82] some describe their visions pictorially, and there exist examples of visionary art which are 'salvia-inspired'. Others claim musical inspiration from the plant: including "Salvia divinorum" by 1200 Micrograms, "Salvia" by Deepwater Sunshine, and "Flight 77" by Paul Dereas.[82]
Cautionary notes
Dale Pendell expresses some concerns about the use of highly concentrated forms of salvia. In its natural form salvia is more balanced and benevolent, and quite strong enough, he argues. High strength extracts on the other hand can show "a more precipitous, and more terrifying, face" and many who try it this way may never wish to repeat the experience.[83]
The Salvia divinorum User's Guide hosted on Daniel Siebert's website recommends having a trip sitter present to those who are new to salvia, are experimenting with a stronger form, or are using a more effective method of ingestion.
An experienced salvia user who is chewing a quid, may often choose to do it alone, and may be quite safe in doing so. But having a pleasant, sensible, sober sitter is an absolute must if you are trying vaporization, smoking high doses of extract-enhanced leaves, or using pure salvinorin.The guide says that while the effects of salvia are generally quite different from those of alcohol, like alcohol, it impairs coordination. It also emphasizes that salvia is not a 'party drug.'
Salvia is not 'fun' in the way that alcohol or cannabis can be. If you try to party with salvia you probably will not have a good experience. Salvia is a consciousness-changing herb that can be used in a vision quest, or in a healing ritual. In the right setting, salvia makes it possible to see visions. It is an herb with a long tradition of sacred use. It is useful for deep meditation. It is best taken in a quiet, nearly dark room; either alone, or with one or two good friends present.After-effects
Short term
After the peak effects, normal awareness-of-self and the immediate surroundings return but lingering effects may be felt. These short-term lingering effects have a completely different character than the peak experience. About half of users report a pleasing 'afterglow', or pleasant state of mind following the main effects. Researchers from the University of California and California Pacific Medical Center Research Institute conducted a survey of 500 salvia users which identified that they 'sometimes or often' experience certain effects, including:[84]
Increased insight: 47% Decreased insight: 1.8% Improved mood: 44.8% Worsened mood: 4.0% Increased connection with Universe or Nature: 39.8% Decreased connection with Universe or Nature: 5.4% Increased sweating: 28.2% Decreased sweating: 1.6% Body felt warm or hot: 25.2% Body felt cold: 6.4% Increased self-confidence: 21.6% Decreased self-confidence: 2.4% Improved concentration: 19.4% Difficulty concentrating: 12.0% Other commonly reported effects include:
- Feelings of calmness: 42.2%
- Weird thoughts: 36.4%
- Things seeming unreal: 32.4%
- Floating feelings: 32%
- Mind racing: 23.2%
- Feeling lightheaded: 22.2%
Long term
Differing studies suggest no overall consensus so far with regard to the long-term effects of Salvia divinorum on mood. It is well-established that some k-opioid agonists can cause dysphoria in humans,[85] and research using rats in forced-swim tests has been used to suggest that Salvia divinorum may have "depressive-like" effects.[86] However, a report has been published detailing an individual case of Salvia divinorum use as self-medicated treatment for depression,[87] and Baggott's survey of 500 people with firsthand experience of salvia found that 25.8% of respondents reported improved mood and "antidepressant-like effects" lasting 24 hours or longer. Only 4.4% reported persisting (24 hours or more) negative effects (most often anxiety) on at least one occasion.[79]
There has been one report of salvia precipitating psychosis. However, the authors suspected that their patient was already genetically predisposed to schizophrenia.[88]
It has been suggested that the long-term effects of salvia use may include feelings of déjà vu.[89]
The Baggott survey found little evidence of addictive potential (chemical dependence) in its survey population. 0.6% percent of respondents reported feeling addicted to or dependent on salvia at some point, and 1.2% reported strong cravings. About this the researchers said "there were too few of these individuals to interpret their reports with any confidence".
Most users report no hangover or negative after-effects (e.g. withdrawal, comedown or rebound effect) the next day. This is consistent with the apparent low toxicity of salvia indicated by research conducted at the University of Nebraska.[13]
Therapeutic potential
Aside from individual reports of self-medicated use in the treatment of depression,[87][90] research suggests that Salvia divinorum, in line with the studied effects of other κ-opioid agonists,[91] may have further therapeutic potential.
Thomas Prisinzano, assistant professor of medicinal and natural products chemistry at the University of Iowa, has suggested that salvia may help treat cocaine addiction:
You can give a rat free access to cocaine, give them free access to Salvinorin A, and they stop taking cocaine.Professor Bryan L. Roth, director of the National Institute on Mental Health's Psychoactive Drug Screening Program, has said:
We think that drugs derived from the active ingredient could be useful for a range of diseases: Alzheimer's, depression, schizophrenia, chronic pain and even AIDS or HIV.Clinical pharmacologist John Mendelsohn has also said:
There may be some derivatives that could be made that would actually be active against cancer and HIV [...] At the present time, there are a lot of therapeutic targets that have many people excited.An ABC news story which reported on this went on to suggest "the excitement could vanish overnight if the federal government criminalized the sale or possession of salvia, as the Drug Enforcement Agency is considering doing right now."[90] A proposed Schedule I classification would mean (among other things) that there's no "currently accepted medical use" as far as the United States government is concerned.[92] Scientists worry that such legislation would restrict further work.[93][94] Mendelsohn said scheduling salvia could scare away a great deal of research and development into salvia's therapeutic promise.[90]
Controversy
The relatively recent emergence of Salvia divinorum in modern Western culture, in comparison to its long continuing traditions of indigenous use, contrasts widely differing attitudes on the subject.
Opinions range from veneration of the plant as a spiritual sacrament or "a gift from the gods",[15][95] to 'threat to society', to be banned as quickly as possible in order to "spare countless families the horror of losing a loved one to the relentless tentacles of drug abuse".[96]
Media coverage
Interest in Salvia divinorum has been escalating in the news media, particularly in the United States, where an increasing number of newspaper reports have been published and television news stories broadcast.[97]
These stories generally raise alarms over salvia's legal status. Headlining for example with comparisons to LSD,[98][99][100] or describing it as "the new pot"[101] for instance, with parental concerns being raised by particular focus on salvia's use by younger teens.
Story headlines may also include 'danger' keywords, such as "Dangerous Herb is Legal..."[102] or "Deadly Dangers Of A Street Legal High".[103]
Mainstream news coverage and journalistic opinion has widely been negative on the subject. In a local news report aired on ABC affiliate WJLA in Washington, DC on July 11, 2007, the anchors are seen to exchange expressions of incredulity when referring to a salvia story with the following introduction "Now, an exclusive I-Team investigation of a hallucinogenic drug that has begun to sweep the nation. What might amaze you is that right now the federal government is doing nothing to stop it".[104]
In March 2008 a Texas news report aired with the story "A legal drug that teenagers are now using to get high could soon be banned here in San Antonio - all because of a Fox News 4 investigation", going on to say, "The drug is legal in Texas, at least for now. But a News 4 investigation could lead to a new ordinance to protect your kids."[105]
Many salvia media stories headline with comparisons to LSD. However, while LSD and salvia's active constituent salvinorin A may have comparative potencies, in the sense that both can produce their effects with low dosage amounts, they are otherwise quite different. The two substances are not chemically similar or related, as salvinorin A is found naturally in a single plant while LSD is chemically semisynthesized from lysergamides like ergotamine. They are ingested in different ways and produce different effects, which manifest themselves over different timescales. For example, the effects of salvia when smoked typically last for only a few minutes as compared to LSD, whose effects can persist for 8–12 hours.[69][106]
Brett's law
Main article: Brett's lawA particular focus of many US media stories is the long-running coverage of the case of Brett Chidester.[104][107] Chidester was a 17 year old Delaware student who committed suicide in January 2006 by carbon monoxide poisoning.
Reportedly, some months before this, Brett's mother Kathleen Chidester had learned about his salvia use and questioned him about it. Brett said that he had ceased his experimentation, but his parents do not believe that he was telling the truth. They have instead argued that salvia-induced depression was largely to blame for his death. Some of Brett's earlier writings about his salvia experiences have been used to suggest that it made him think "existence in general is pointless." Some media stories have referred to these earlier written experience reports as if they were part of Brett's suicide note.[94][107] In any case, a law was soon passed in Delaware classifying the herb as a Schedule I controlled substance in that state. This legislation was nicknamed Brett's law (formally referred to as Senate bill 259).[108]
Although the Chidester story has been given continued exposure by US media, there has not been anywhere else, either before or since this controversial incident, any other reported cases involving or alleging Salvia divinorum as a serious factor in suicide, overdose, accidental, or any other kind of death. Regarding this, San Francisco attorney Alex Coolman has commented, "It's remarkable that Chidester's parents, and only Chidester's parents, continue to be cited over and over again by the mainstream media in their coverage of the supposed 'controversy' over the risks of Salvia divinorum."[109]
Kathleen Chidester has continued campaigning for "Schedule I"-like legislation beyond their home state of Delaware. For example, three years after Brett's death, in written testimony in support of Senator Richard Colburn's proposed Senate Bill to the Maryland State Legislature, saying, "My hope and goal is to have salvia regulated across the US. It's my son's legacy and I will not end my fight until this happens."[110]
Usage shown on YouTube
A reported concern has been the emergence of YouTube videos showing alleged salvia users laughing uncontrollably, apparently unable to perform simple tasks or to communicate.[104][111] In an interview with California-based newspaper the San Francisco Chronicle, published in June 2007, Daniel Siebert was quoted as saying:
"Those videos are certainly not going to help the situation. They make salvia look like some horrible drug that makes people nuts and dangerous [...]" and "The sad thing is it creates this public image where people don't realize there are sensible ways to use something like this."[112]
The New York Times has reported that "in state after state [...] the YouTube videos have become Exhibit A in legislative efforts to regulate salvia."
Waco Representative Charles Anderson (R), who is sponsoring one of several bills to ban salvia in Texas saying, "When you see it, well, it sure makes a believer out of you." Anderson told colleagues at a legislative hearing about a video that depicts a salvia user behind the wheel of a car.[nb 4]
"What we really worry about," said Mr. Anderson at the hearing, "is youngsters doing this and then getting in a vehicle or getting on a motorcycle or jumping in a pool somewhere."[113]
Michigan Representative Michael Sak (D) submitted a bill which proposed Schedule I classification of Salvia divinorum and salvinorin A.[114] He said that if people had questions about the deleterious affects of salvia, they should go on YouTube to watch the videos. A reporter questioned Sak as to whether he had ever seen a "Girls Gone Wild" video, and whether that would incite him to make alcohol illegal (Sak replied that he hadn't yet had a chance to review the material).[nb 5]
Nebraska Senator Vickie McDonald said:
"Anytime anything's on YouTube it's an issue," and "Legislators, parents, grandparents, we need to be on top of these things," [...] "We need to protect our children..."[117][118]
Senator McDonald of Nebraska proposed Schedule I listing Salvia divinorum as part of their Controlled Substances Act, under which possession of salvia would have been considered a Class IV felony with a penalty of up to five years and trafficking would have fallen under a Class III felony with up to a 20 year penalty.[118]
In Massachusetts, YouTube videos were shown by a retired police officer to public health and judiciary committees as evidence in favor of outlawing it there.[119]
The issue has been raised of whether the salvia videos are in breach of YouTube's own community guidelines, which ask users not to "cross the line" and post videos showing "bad stuff" like "drug abuse".[120] The question is considered as particularly problematical as the videos may be something of an enforcement grey area.[121]
Legal status
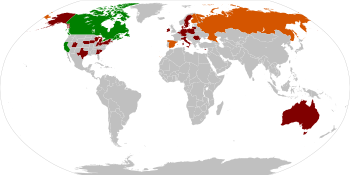
Main article: Legal status of Salvia divinorumAt present Salvia divinorum remains legal in most countries. Countries where salvia is controlled in some manner include: Australia, Belgium, Canada, Denmark, Estonia, Finland, Italy, Japan, the United States, Russia, Spain, and Sweden.[16][122][123]
The prohibitive degree of Salvia divinorum legislation varies widely from country to country. Australia has imposed its strictest 'schedule 9' (US Schedule I equivalent), and Italy has also placed salvia in its 'Table I' of controlled substances (also US Schedule I equivalent). In Spain, there are controls focusing only on the commercial trade of Salvia divinorum, personal cultivation (i.e. for non-commercial use) is not targeted.[122][123]
Salvia legislation may prove difficult to enforce. The plant has a nondescript appearance; unlike many other drug plants, the leaves are non-descript, and without a detectable odour. Salvia divinorum can be grown as an ordinary houseplant without the need of special equipment such as hydroponics or high-power lights.[124][125][nb 1]
UK legality
In the United Kingdom, following a local newspaper story in October 2005,[126] a parliamentary Early Day Motion was raised calling for Salvia divinorum to be banned there. However, it only received 11 signatures.[127] A second motion was raised in October 2008 attracting 18 signatures,[128] with it being reported that Mann had also written to the then Home Secretary Jacqui Smith. The Advisory Council on the Misuse of Drugs, the independent body that advises UK government on drugs, was asked to investigate further.[129]
US legality
Main article: Legal status of Salvia divinorum in the United StatesNational legislation for amendment of the Controlled Substances Act to place salvinorin A and Salvia divinorum in Schedule I at the federal level in the United States was proposed in 2002 by Representative Joe Baca (D- California). Those opposed to bill HR 5607 include Daniel Siebert, who sent a letter to Congress arguing against the proposed legislation,[130] and the Center for Cognitive Liberty and Ethics (CCLE), who sent key members of the US Congress a report on Salvia divinorum and its active principle,[4] along with letters from an array of scientists who expressed concern that scheduling Salvia divinorum would negatively impact important research on the plant. The bill did not pass.[131][132][133]
Although salvia is not regulated under the Controlled Substances Act, some American states, including Alabama, Delaware, Louisiana, Michigan, Missouri, Ohio and others, have passed their own laws.[16][134] Several other states have proposed legislation against salvia, including Alaska, California, Florida, Iowa, Maryland, New Jersey, New York, Oregon, Pennsylvania, and Texas. Many of these proposals have not made it into law, with motions having failed, stalled or otherwise died, for example at committee review stages.[122][123]
Where individual state legislation does exist, it varies from state to state in its prohibitive degree.[122]
Salvia divinorum has been banned by various branches of the U.S. military and some military bases.[135][136][137][138]
Internet sale
Some internet vendors will not sell live salvia cuttings, leaf, or leaf products to states where its use is restricted or prohibited. [139]
Per their drugs and drug paraphernelia policy, eBay does not permit sale of Salvia divinorum or derived products (despite legality in most areas).[140]
Opinions and arguments
Main article: Legal status of Salvia divinorum#Opinions and argumentsConcerns expressed by some politicians on the subject of Salvia reflect those of the media, with comparisons to LSD and particular focus on "protecting our children" being echoed;[98][141][142] and with legislative proposals following soon after news stories breaking.[102][105][143][144][145]
Some arguments against Salvia have been of a preventative nature, "We need to stop this before it gets to be a huge problem not after it gets to be a huge problem,"[146] or of an imitative nature, "The Australians have clearly found a problem with it. There's obviously a risk in people taking it."[126] Concerns about driving while under the influence of Salvia have also been expressed.[147][148]
Opponents of more prohibitive measures against Salvia argue that such reactions are largely due to an inherent prejudice and a particular cultural bias rather than any actual balance of evidence, pointing out inconsistencies in attitudes toward other more toxic and addictive drugs such as alcohol and nicotine.[149][nb 6][nb 7] While not objecting to some form of legal control, in particular with regard to the sale to minors or sale of enhanced high-strength extracts, most salvia proponents otherwise argue against stricter legislation.[122]
Those advocating consideration of Salvia divinorum's potential for beneficial use in a modern context argue that more could be learned from Mazatec culture, where salvia is not really associated with notions of drug taking at all and it is rather considered as a spiritual sacrament. In light of this it is argued that Salvia divinorum could be better understood more positively as an entheogen rather than pejoratively as a hallucinogen.[154][nb 8]
Public opinion
Despite its growing notoriety in some circles, media stories generally suggest that the public at large are still mostly unaware of salvia, with the majority perhaps having never even heard of it.[107]
Although published responses may not necessarily be representative of public opinion as a whole, some news agencies generally support reader and viewer feedback in connection with their stories.[96][98][105][107][158]
Notes
- ^ a b The case of North Dakota resident Kenneth Rau is reported as likely being the first person charged for Salvia divinorum possession in the United States.[17][18] A Nebraska store owner was also charged for selling salvia, but not under the auspices of any specific law against Salvia divinorum. Christian Firoz was instead charged under a general Nebraskan statute where it is illegal to sell a product to induce an intoxicated condition. The jury in the Firoz case returned a verdict of not guilty.[19] The Rau case did not go to jury trail. Rau pleaded guilty to the charge of Class C felony possession of salvia, and also Class A misdemeanor possession of drug paraphernalia and Class B misdemeanor possession of marijuana. He received a deferred imposition of sentence. He will not go to prison, but will be on supervised probation for three years; the charges will be removed from his record if he successfully completes his time on supervision. The judge ordered Rau to complete a chemical dependency evaluation and any recommended treatment and pay $575 in court fees.[20] - See also the North Dakota detail under the main article on the Legal status of Salvia divinorum (and, re the Christian Firoz case, the Nebraska detail).
- ^ Botanist Valdés (1987), wrote that, "It is doubtful that the Salvia is a true cultigen", based partly on his belief that it was first "collected in the highlands and planted in more accessible places, where it becomes naturalized". The main cultigen proponent is Gordon Wasson, who is not a botanist.
- ^ Reisfield is unsure why Epling "used the invalid name (the synonym rather than the valid name S. concolor), nor why he considered this species close to S. divinorum".
- ^ In fact the video is one in a series of parodies featuring Erik J. Hoffstad, a production assistant in Los Angeles. In the film Hoffstad smokes salvia in a parked car (his friends reportedly making sure he did not have the real keys).[113]
- ^ The reporter noted that Sak had received more money from the Michigan Beer and Wine Wholesalers Association than anyone else in the House.[115] According to the National Institute on Money in State Politics, the Michigan Beer and Wine Wholesalers Association was the highest contributor to Zak's 2006 political campaign.[116]
- ^ According to the National Institute on Money in State Politics, which indicates the major sources of campaign contributions for U.S. politicians, a large part of contributions to U.S. Congressional campaigns comes from alcohol and tobacco industries.[150] For example, Oregon Representative John Lim"s third largest individual campaign sponsor in 2006 was the Oregon Beer & Wine Distributors Association[116] Lim(R) argued for Schedule I classification of Salvia in Oregon. Former Delaware Senator Karen Peterson's second largest group campaign donations in 2006 came from "Beer, Wine & Liquor" industries.[116] Peterson(D) introduced Schedule I classification of Salvia divinorum in Delaware. Senator Tim Burchett(R) sponsored Salvia legislation in Tennessee. In 2006, his second largest individual campaign donation came from the Tennessee Malt Beverage Association.[116] In the same period alcohol and tobacco related contributions amounted to the fourth largest industry contributions for Utah Representative Paul Ray(R).[116] Representative Ray submitted a bill calling for Schedule I classification of Salvia in that state.[143] Alcohol related contributions also featured highly for Illinois Representative Dennis Reboletti(R) "Beer, Wine & Liquor" was his ninth highest industry contributor in 2006 and 2008.[116] Representative Reboletti sponsored a bill for Schedule I classification of Salvia divinorum in Illinois.[151]
- ^ The worldwide number of alcohol-related deaths is calculated at over 2,000 people per day,[152] in the US the number is over 300 deaths per day.[153]
- ^ Other entheogenic plants with continuing traditions principally of spiritual use include peyote (and other psychoactive cacti), iboga, virola, ayahuasca, and various types of psychoactive fungi.[155] Current U.S. Federal Law allows two of these to be used in a spiritual context. The Native American Church is permitted use of peyote; the Uniao do Vegetal (or UDV) is permitted use of ayahuasca.[156] Although not consistently granted (varying from state to state), the principal grounds for such concessions are constitutional,[157] with further grounds following from the Religious Freedom Restoration Act.
Citations
- ^ a b c d e f g Valdés, Díaz & Paul 1983, p. 287.
- ^ Medana et al. 2005, p. 131.
- ^ a b Valdés, Díaz & Paul 1983, p. 288.
- ^ a b c Boire 2002.
- ^ a b c d Reisfield 1993, Introduction.
- ^ a b c Valdes 1987, p. 106.
- ^ a b Marushia 2002, p. 3.
- ^ a b c d e Reisfield 1993, The Barrier to Fertility.
- ^ Prisinzano 2006, p. 527.
- ^ a b Imanshahidi & Hosseinzadeh 2006, p. 430.
- ^ a b c d Zhang et al. 2005, p. abstract.
- ^ a b Seeman et al. 2009.
- ^ a b c Mowry, Mosher & Briner 2003, p. 382.
- ^ Grundmann 2007.
- ^ a b c d Valdés, Díaz & Paul 1983.
- ^ a b c DEA 2008.
- ^ a b DRCNet 2008-04-25 (US Media).
- ^ a b Michael 2008-08-03 (US Media).
- ^ a b DRCNet 2009-01-31 (US Media).
- ^ a b Michael 2009-04-22 (US Media).
- ^ a b c Marushia 2002, p. 6.
- ^ a b Marushia 2002, p. 2.
- ^ Valdés, Díaz & Paul 1983, p. 290.
- ^ a b Marushia 2002, p. 11.
- ^ Wasson 1963.
- ^ Valdés 2001.
- ^ a b Ott 1995.
- ^ Dweck 1997, p.15.
- ^ Erowid (Cacahuaxochitl) 2007.
- ^ Kintzios 2000
- ^ Sutton 2004
- ^ Grof 2001
- ^ Honeycutt 2009-02-09 (US Media)
- ^ Mason 2009-01-30 (US Media).
- ^ “Salvia divinorum: effects and use among YouTube users, Cornell Info 2040 - Networks,” http://expertvoices.nsdl.org/cornell-info204/2010/04/22/salvia-divinorum-effects-and-use-among-youtube-users/.
- ^ “Mind Hacks: The YouTube drug observatory,” http://www.mindhacks.com/blog/2010/04/the_youtube_drug_obs.html.
- ^ Lange, J. E.; Daniel, J.; Homer, K.; Reed, M. B.; Clapp, J. D. (2010). "Salvia divinorum: Effects and use among YouTube users". Drug and Alcohol Dependence 108 (1-2): 138–140. doi:10.1016/j.drugalcdep.2009.11.010. PMID 20031341.
- ^ a b Giroud 2000.
- ^ a b c d e Clebsch & Barner 2003, p. 106.
- ^ Reisfield 1993, Previous Research.
- ^ a b c Ott 1996
- ^ Marushia 2002, p. 4.
- ^ Reisfield 1993, Distribution, Ecology, & Flower Initiation.
- ^ a b Jenks, Aaron A.; Walker, Jay B.; Kim, Seung-Chul (2010). "Evolution and origins of the Mazatec hallucinogenic sage, Salvia divinorum (Lamiaceae): a molecular phylogenetic approach". Journal of Plant Research. doi:10.1007/s10265-010-0394-6. PMID 21125306.
- ^ Reisflield 1987, p. 199.
- ^ Foley 1993, p. 56.
- ^ Erowid, Salvia Timeline.
- ^ Siebert 2003.
- ^ Siebert, 'Luna' Clone.
- ^ Siebert (Cultivation advice).
- ^ Beifuss 1997.
- ^ "Growing your OWN Salvia divinorum Seeds: A Simple Step by Step Illustrated Guide". http://members.cox.net/sageseeds/. Retrieved 2011-02-10.
- ^ a b c Prisinzano 2006, p. 528.
- ^ Harding, Schmidt & Tidgewell 2006, p. 107.
- ^ Siebert 2004.
- ^ Kunkel 2004.
- ^ a b Imanshahidi & Hosseinzadeh 2006, p. 431.
- ^ Greiner T, Burch NR, Edelberg R (1958). "Psychopathology and psychophysiology of minimal LSD-25 dosage; a preliminary dosage-response spectrum". AMA Arch Neurol Psychiatry 79 (2): 208–10. PMID 13497365.
- ^ Roth et al. 2002, p. abstract.
- ^ Harding 2005.
- ^ Bigham et al. 2003.
- ^ Munro & Rizzacasa 2003.
- ^ .Capasso 2006.
- ^ Masis 2007-02-28 (US Media)
- ^ Prisinzano, Tidgewell & Harding 2005.
- ^ .Capasso 2008.
- ^ a b c d e Sage Student - Sd User Guide.
- ^ Harrison. The Leaves of the Shepherdess. 2000..
- ^ a b c Siebert (FAQ—Section VI).
- ^ Siebert (FAQ—Section X).
- ^ Valdes 1983.
- ^ SAMHSA 2006.
- ^ a b Sullum 2009.
- ^ a b Siebert (Smoke advice).
- ^ Siebert (FAQ—Section IV).
- ^ Siebert 1994.
- ^ Turner 1996.
- ^ Babu 2008.
- ^ a b Baggott & Erowid 2004, p. 14.
- ^ Mercury House Publishing Online.
- ^ Lizard 2001.
- ^ a b Siebert (Arts)
- ^ Pendell 1995.
- ^ Baggott & Erowid 2004, p. 12.
- ^ Rothman et al. 2000, p. abstract
- ^ Carlezon, Béguin & DiNieri 2005.
- ^ a b Hanes 2001, p. 634–635.
- ^ Przekop 2009.
- ^ Singh 2006
- ^ a b c Terry 2007-10-03 (US Media).
- ^ Schenk 2001, p. 629–34.
- ^ DEA 2002, title 21, section 812.
- ^ Roth 2007.
- ^ a b Schaper 2006-03-20 (US Media).
- ^ Schultes 1992.
- ^ a b Cardall 2006-12-12 (US Media).
- ^ Shafer 2008-05-06 (US Media).
- ^ a b c Martell 2007-06-18 (US Media).
- ^ Devine 2007-02-19 (US Media).
- ^ Blake 2006-11-13 (US Media).
- ^ Sanchick 2007-02-14 (US Media).
- ^ a b Dujanovic 2006-11-27 (US Media).
- ^ Quinones 2006-11-30 (US Media).
- ^ a b c Baskin 2007-07-11 (US Media).
- ^ a b c Chancellor 2008-03-14 (US Media).
- ^ Shulgin 1997.
- ^ a b c d Anderson 2006-04-13 (US Media).
- ^ Peterson 2006.
- ^ Coolman 2007.
- ^ Michael 2009-01-28 (US Media).
- ^ Sontaya 2007-05-10 (US Media).
- ^ Allday 2007-06-27 (US Media).
- ^ a b Sack 2008-09-08 (US Media).
- ^ Sak 2008.
- ^ McNamara 2008-04-30 (US Media).
- ^ a b c d e f MiSP 2006.
- ^ White 2008-01-08 (US Media).
- ^ a b Berry 2008-01-07 (US Media).
- ^ Sege 2008-07-22 (US Media).
- ^ YouTube Guidelines 2008.
- ^ Sarno 2008-09-12 (US Media).
- ^ a b c d e Siebert (Legal status).
- ^ a b c Erowid (Legal status).
- ^ Shulgin 2003.
- ^ Chalmers 2006-05-06 (US Media).
- ^ a b Worksop 2005-10-14 (UK Media).
- ^ Mann 2005.
- ^ Mann 2008.
- ^ Doward 2009-04-26 (UK Media).
- ^ Siebert 2002.
- ^ Baca 2002.
- ^ DEA 2003.
- ^ CCLE 2002.
- ^ "Code of Alabama 1975". Alabama Legislature. p. Section 13A-12–214.1. http://alisondb.legislature.state.al.us/acas/CodeOfAlabama/1975/coatoc.htm. Retrieved 17 March 2011.
- ^ "Erowid Salvia Divinorum Vault : Legal Status". Erowid.org. http://www.erowid.org/plants/salvia/salvia_law.shtml. Retrieved 2011-05-01.
- ^ http://www.erowid.org/plants/salvia/salvia_law3.pdf
- ^ Sheri Kangas (2007-09-19). "Hallucinogenic Salvia illegal for military members". .hurlburt.af.mil. http://www2.hurlburt.af.mil/news/story.asp?id=123068724. Retrieved 2011-05-01.
- ^ "Military Cracks Down on 'Legal High' From Salvia Divinorum". Aolnews.com. 2010-02-17. http://www.aolnews.com/nation/article/military-cracks-down-on-legal-highs/19360724. Retrieved 2011-05-01.
- ^ Hoyle 2008-03-12 (US Media).
- ^ eBay Policy 2009.
- ^ Clark 2007-03-05 (US Media).
- ^ Reboletti 2007 (Mar).
- ^ a b Dujanovic 2006-11-28 (US Media).
- ^ Sanchick 2007-02-15 (US Media).
- ^ Eckenrode 2007-03-08 (US Media).
- ^ KXMBTV 2007-01-31 (US Media).
- ^ NBC10 2006-04-11 (US Media).
- ^ Smith 2007-09-25 (US Media).
- ^ Nutt et al. 2007.
- ^ Follow The Money, Industry Influence.
- ^ Reboletti 2007 (Jan), full text - p.7.
- ^ Lopez 2005, Table 2.
- ^ NIAAA 2001.
- ^ Blosser (Mazatec Lessons).
- ^ see peyote, iboga, virola, ayahuasca, etc.
- ^ see Native American Church and Uniao do Vegetal.
- ^ Madison 1789.
- ^ Tompkins 2007-07-13 (US Media).
References
- Babu, Kavita M.; McCurdy, Christopher R.; Boyer, Edward W. (2008). "Opioid receptors and legal highs: Salvia divinorum and Kratom". Clinical Toxicology 46 (2): 146–152. doi:10.1080/15563650701241795. PMID 18259963. http://informahealthcare.com/doi/abs/10.1080/15563650701241795.
- Baca, Rep. Joe (October 2002). "To amend the Controlled Substances Act to place Salvinorin A in Schedule I (H.R.5607)". Bills, Resolutions. The Library of Congress (THOMAS). http://thomas.loc.gov/cgi-bin/bdquery/z?d107:h.r.05607:. Retrieved 2007-10-14.
- Baggott, Matthew; Erowid, E. & F. (June 2004). "A Survey of Salvia divinorum Users" (PDF). Erowid Extracts 6: 12–14. http://www.erowid.org/general/newsletter/erowid_newsletter6.pdf. Retrieved 2007-05-04.
- Beifuss, Will (Summer 1997). "Cultivating Diviner's Sage - A step by step guide to cultivation, propagation, and keeping your Salvia plants happy". Issue 1. The Resonance Project. http://www.erowid.org/plants/salvia/salvia_cultivation1.shtml. Retrieved 2009-11-16.
- Bigham, Andrea K.; Munro, Thomas A.; Rizzacasa, Mark A.; Robins-Browne, Roy M. (July 2003). "Divinatorins A-C, New Neoclerodane Diterpenoids from the Controlled Sage Salvia divinorum." (PDF). Journal of Natural Products 66 (9): 1242–1244. doi:10.1021/np030313i. PMID 14510607. http://www.sagewisdom.org/divinatorinsa-c.pdf. Retrieved 2007-06-25.
- Blosser, Brett. "Lessons in The Use of Mazatec Psychoactive Plants". The Salvia divinorum Research and Information Center. http://www.sagewisdom.org/lessons.html. Retrieved 2007-10-19.
- Boire, Richard Glen; Russo, Ethan & Fish, Adam Richard et al. (October 2002). "Salvia divinorum ~ Information Concerning the Plant and its Active Principle—(re. H.R. 5607)". The Salvia divinorum Defense Fund. Center for Cognitive Liberty & Ethics (CCLE). http://www.cognitiveliberty.org/dll/salvia_rpt.html. Retrieved 2007-10-16.
- Burchett, Sen. Tim (2006). "Senate Bill No. 3247" (PDF). Public Acts, 2006, Chapter No. 700. The General Assembly the State of Tennessee. http://tennessee.gov/sos/acts/104/pub/pc0700.pdf. Retrieved 2007-10-16.
- Capasso, R.; Borrelli, F.; Capasso, F.; Siebert, D. J.; Stewart, D. J.; Zjawiony, J. K.; Izzo, A. A. (2006). "The hallucinogenic herb Salvia divinorum and its active ingredient salvinorin A inhibit enteric cholinergic transmission in the guinea-pig ileum.". Neurogastroenterology & Motility 18 (1): 69–75. doi:10.1111/j.1365-2982.2005.00725.x. PMID 16371085. http://pt.wkhealth.com/pt/re/negm/abstract.00043897-200601000-00009.htm;jsessionid=KJjQJhL2NFGc2FSXW12GyhLB0w5hfQnCbLPkZbqdrMQn0tjnpTWr!-1601773455!181195629!8091!-1.
- Capasso, R.; Borrelli, F.; Zjawiony, J. K.; Kutrzeba, L.; Aviello, G.; Sarnelli, G.; Capasso, F.; Izzo, A. A. (2008). "The hallucinogenic herb Salvia divinorum and its active ingredient salvinorin A reduce inflammation-induced hypermotility in mice.". Neurogastroenterology & Motility 20 (2): 142–148. http://pt.wkhealth.com/pt/re/negm/abstract.00043897-200802000-00007.htm;jsessionid=KJyBbn620YJ26l6qmrv7p9M1kQL4yFC8Gq1y86TrgfJ5Nr0yVzv5!136633189!181195628!8091!-1.
- Carlezon, William A.; Béguin, Cécile; DiNieri, Jennifer A.; Baumann, MH; Richards, MR; Todtenkopf, MS; Rothman, RB; Ma, Z et al. (October 2005). "Depressive-Like Effects of the κ-Opioid Receptor Agonist Salvinorin A on Behavior and Neurochemistry in Rats". Journal of Pharmacology and Experimental Therapeutics 316 (1): 440–447. doi:10.1124/jpet.105.092304. PMID 16223871. http://jpet.aspetjournals.org/cgi/content/abstract/316/1/440. Retrieved 2007-05-04.
- CCLE (2002). "HR 5607 Archive". The Entheogens and Drug Policy Project. Center for Cognitive Liberty & Ethics. http://www.cognitiveliberty.org/dll/hr5607_archive.htm. Retrieved 2007-10-14.
- Clebsch, Betsy; Carol D. Barner (2003). The New Book of Salvias. Timber Press. ISBN 9780881925609. http://books.google.com/?id=NM0iwB8GrQYC&pg=PA106.
- Coolman, Alex (2007). "Brett Chidester and Salvia: "Suicide Solution" Redux". Drug Law, Policy and Politics in California, the Ninth Circuit, and the United States. Archived from the original on 2008-01-17. http://web.archive.org/web/20080117105446/http://druglaw.typepad.com/drug_law_blog/2007/10/on-brett-chides.html. Retrieved 2009-07-26.
- DEA (US Dept. Justice) (January 2002). "21 USC Sec. 812 01/22/02". Controlled Substances Act—Title 21—Food and Drugs Chapter 13—Drug Abuse Prevention and Control Subchapter I—Control and Enforcement Part B—Authority To Control; Standards and Schedules—Section 812. Schedules of controlled substances. U.S. Drug Enforcement Administration (DEA). Archived from the original on September 30, 2007. http://web.archive.org/web/20070930064509/http://www.usdoj.gov/dea/pubs/csa/812.htm. Retrieved 2007-10-06.
- DEA (US Dept. Justice) (June 2003). "Information Bulletin: Salvia Divinorum". Microgram Bulletin (Office of Forensic Sciences Washington, D.C.: U.S. Drug Enforcement Administration (DEA)) XXXVI (6). http://www.usdoj.gov/dea/programs/forensicsci/microgram/mg0603/mg0603.html. Retrieved 2007-10-14.
- DEA (US Dept. Justice) (June 2008). "Salvia Divinorum and Salvinorin A". Drugs and Chemicals of Concern. Office of Diversion Control—U.S. Drug Enforcement Administration (DEA). http://www.deadiversion.usdoj.gov/drugs_concern/salvia_d/salvia_d.htm. Retrieved 2008-09-15.
- Diaz, J.L. (April 1977). "Ethnopharmacology of Sacred Psychoactive Plants Used by the Indians of Mexico". Annual Review of Pharmacology and Toxicology (Annual Reviews) 17: 647–675. doi:10.1146/annurev.pa.17.040177.003243. PMID 17363.
- Dweck, Anthony C. (1997) Chapter One: The Folklore and Cosmetic Use of Various Salvia Species. Retrieved July 21, 2007.
- Erowid. "Salvia Legal Status". Erowid. http://www.erowid.org/plants/salvia/salvia_law.shtml. Retrieved 2007-10-14.
- eBay (2009). "Drugs and drug paraphernalia policy". eBay. http://pages.ebay.com/help/policies/drugs-drug-paraphernalia.html. Retrieved 28 November 2009.
- Erowid (August 2007). "Cacahuaxochitl". http://www.erowid.org/plants/cacahuaxochitl/cacahuaxochitl.shtml. Retrieved 2007-08-16.
- Foley, Michael J.; Hedge, Ian; Moller, Michael (2008). "The enigmatic Salvia tingitana (Lamiaceae): a case study in history, taxonomy and cytology". Willdenowia 38: 41–59. doi:10.3372/wi.38.38102. ISSN 0511-9618. http://www.bgbm.org/willdenowia/w-pdf/wi38-1Foley+al.pdf. Retrieved 2009-04-02.
- Giroud, C.; Felber F., Augsburger M. (2000). "Salvia divinorum: an hallucinogenic mint which might become a new recreational drug in Switzerland". Forensic Science International 112 (2): 143–150. doi:10.1016/S0379-0738(00)00180-8. PMID 10940599. http://www.sagewisdom.org/giroudetal.pdf.
- Grof, Stanislav (Fall 2001). "Stanislav Grof interviews Dr. Albert Hofmann Esalen Institute, Big Sur, California, 1984". Maps: Multidisciplinary Association for Psychedelic Studies XI (2). http://www.maps.org/news-letters/v11n2/11222gro.html.
- Grundmann (2007). "Salvia divinorum and Salvinorin A: An Update on Pharmacology and Analytical Methodology". http://google.com/scholar?q=cache:itG3jcPEUqoJ:scholar.google.com/+salvinorin+a+toxicity&hl=en. Retrieved 2009-10-04.
- Hanes, Karl R. (December 2001). "Antidepressant Effects of the Herb Salvia Divinorum: A Case Report". Journal of Clinical Psychopharmacology 21 (6): 634–635. doi:10.1097/00004714-200112000-00025. PMID 11763023. http://www.sagewisdom.org/jclinpsych.html. Retrieved 2007-05-08.
- Harding, Wayne W; Tidgewell Kevin; Schmidt Matthew; Shah Kushal; Dersch Christina M; Snyder John; Parrish Damon; Deschamps Jeffrey R; Rothman Richard B; Prisinzano Thomas E (2005). Salvinicins A and B, new neoclerodane diterpenes from Salvia divinorum. Organic letters 7(14):3017–20.
- Harding, Wayne W.; Schmidt, Matthew; Tidgewell, Kevin; Kannan, P; Holden, KG; Gilmour, B; Navarro, H; Rothman, RB et al. (January 2006). "Synthetic Studies of Neoclerodane Diterpenes from Salvia divinorum: Semisynthesis of Salvinicins A and B and Other Chemical Transformations of Salvinorin A". Journal of Natural Products 69 (1): 107–112. doi:10.1021/np050398i. PMC 2544632. PMID 16441078. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2544632. Retrieved 2007-03-24.
- Harrison, Kathleen (2000). "The Leaves of the Shepherdess". Sisters of the Extreme. http://www.sagewisdom.org/shepherdess.html. Retrieved 2010-04-03. "The curandero unrolled banana-leaf bundles of hand-sized Salvia divinorum leaves, slightly wilted, and sorted them into pairs... He doled out forty pairs to me, rolled them into a long wad, rather like a salad rolled into a cigar. He explained that after he said the invoking prayers and we stated aloud our intentions, I was to eat the leaves. I was told not to hesitate at their bitterness, not to stop until I had eaten them all"
- Hingson, Ralph; Timothy Heeren, Michael Winter & Henry Wechsler (April 2005). "Magnitude of Alcohol-Related Mortality and Morbidity Among U.S. College Students Ages 18–24: Changes from 1998 to 2001". Annual Review of Public Health 26: 259–279. doi:10.1146/annurev.publhealth.26.021304.144652. PMID 15760289. figures cited by the Marin Institute
- Imanshahidi, Mohsen; Hosseinzadeh, Hossein (April 2006). "The Pharmacological Effects of Salvia species on the Central Nervous System". Phytotherapy Research 20 (6): 427–437. doi:10.1002/ptr.1898. PMID 16619340. http://www3.interscience.wiley.com/cgi-bin/abstract/112593742/ABSTRACT. Retrieved 2007-03-24. "However, when smoked (in a manner similar to free base cocaine), the compound is effective in doses of 200–500 μg and produces visions that last from 30 min to an hour or two, while doses over 2 mg are effective for much longer. At doses greater than 500 μg the subject is often no longer aware of their surroundings and may enter an uncontrollable delirium. This compound is the most potent naturally occurring hallucinogen thus far isolated."
- Kintzios, Spiridon E. (2000). Sage: The Genus Salvia. CRC Press. p. 10. ISBN 9789058230058. http://books.google.com/?id=iE7-nuI9S7UC&pg=PA10.
- Kunkel, Dennis (2004). "Leaf glandular trichome (Salvia divinorum)". Scientific stock photography library of light microscope pictures and electron microscopy images featuring science and biomedical microscopy photos. Dennis Kunkel Microscopy, Inc. Archived from the original on October 31, 2007. http://web.archive.org/web/20071031030340/http://denniskunkel.com/DK/Plants/24071B.html. Retrieved 2009-04-13.
- Lizard (2001). "Green Gnosis (from Salvia Divinorum Inspired Arts)". The Salvia divinorum Research and Information Center. http://www.sagewisdom.org/greengnosis.html. Retrieved 2007-06-28.
- Lopez, Alan D (April 2005). "The evolution of the Global Burden of Disease framework for disease, injury and risk factor quantification: developing the evidence base for national, regional and global public health action". Globalization and Health (BioMed Central Ltd) 1 (5): 5. doi:10.1186/1744-8603-1-5. PMC 1143783. PMID 15847690. http://www.globalizationandhealth.com/content/1/1/5. Table 2. Global burden of disease and injury attributable to selected risk factors, 1990.
- Madison, James et al.. The Bill of Rights, the First Amendment (with regard to the United States Constitution), approved September 25, 1789, ratified December 15, 1791.
- Mann, John (MP) (October 2005). "EDM 796—Salvia divinorum". Early Day Motion. Parliamentary Information Management Systems (pims). http://edmi.parliament.uk/EDMi/EDMDetails.aspx?EDMID=29114&SESSION=875. Retrieved 2007-10-14.
- Marushia, Robin (June 2003). "Salvia divinorum: The Botany, Ethnobotany, Biochemistry and Future of a Mexican Mint" (PDF). Ethnobotany. http://www.cyjack.com/cognition/Salvia.pdf. Retrieved 2007-05-04.
- Medana, Claudio; Massolino, Cristina; Pazzi, Marco; Baiocchi, Claudio (December 2005). "Determination of salvinorins and divinatorins in Salvia divinorum leaves by liquid chromatography/multistage mass spectrometry". Rapid Communications in Mass Spectrometry 20 (2): 131–136. doi:10.1002/rcm.2288. PMID 16331747. http://www3.interscience.wiley.com/cgi-bin/abstract/112163281/ABSTRACT. Retrieved 2007-05-04.
- Mercury House Publishing Home Page. Mercury House Authors: Dale Pendell. Retrieved July 21, 2007.
- Munro, Thomas A.; Rizzacasa, Mark A. (April 2003). "Salvinorins D-F, New Neoclerodane Diterpenoids from Salvia divinorum, and an Improved Method for the Isolation of Salvinorin A" (PDF). Journal of Natural Products 66 (5): 703–705. doi:10.1021/np0205699. PMID 12762813. http://www.sagewisdom.org/salvinorind-f.pdf. Retrieved 2007-06-25. supporting information
- MiSP (2006). "Follow the Money". e. g. Delaware/Peterson, Oregon/Lim, Tennessee/Burchett, Ray/Utah, Illinois/Reboletti. The National Institute on Money in State Politics. http://www.followthemoney.org/Institute/index.phtml. Retrieved 2007-10-16.
- Mowry, Mark; Mosher, Michael; Briner, Wayne (July 2003). "Acute Physiologic and Chronic Histologic Changes in Rats and Mice Exposed to the Unique Hallucinogen Salvinorin A" (PDF). Journal of Psychoactive Drugs 35 (3): 379–382. doi:10.1080/02791072.2003.10400021. PMID 14621136. http://www.sagewisdom.org/mowryetal.pdf. Retrieved 2007-05-04.
- Nance, Rep. John (2006). "House Bill No. 2485". 2nd Session of the 50th Legislature. The State of Oklahoma. http://209.85.135.104/search?q=cache:ZJdvlhU2mRYJ:webserver1.lsb.state.ok.us/2005-06bills/HB/HB2485_CCS.RTF+Salvia+divinorum&hl=en. Retrieved 2007-10-16.
- NIAAA (August 2001). "Number of deaths and age-adjusted death rates per 100,000 population for categories of alcohol-related (A-R) mortality, United States and States, 1979–96.". Database Resources / Statistical Tables. National Institute on Alcohol Abuse and Alcoholism (NIAAA). http://www.niaaa.nih.gov/Resources/DatabaseResources/QuickFacts/Other/armort01.htm. Retrieved 2007-10-20.
- Nutt, David; King, Leslie; Saulsbury, William; Blakemore, Colin (March 2007). "Development of a rational scale to assess the harm of drugs of potential misuse". The Lancet 369 (9566): 1047–1053. doi:10.1016/S0140-6736(07)60464-4. PMID 17382831. http://www.thelancet.com/journals/lancet/article/PIIS0140673607604644/abstract. Retrieved 2007-03-23.
- Ott, Jonathan (1995). "Ethnopharmacognosy and Human Pharmacology of Salvia divinorum and Salvinorin A". Curare 18 (1): 103–129. http://www.erowid.org/references/refs_view.php?A=ShowDoc1&ID=6810. Retrieved 2007-08-16.
- Ott, Jonathan (1996). "Psychoactive Card IV. Salvia divinorum Epling et Játiva: Leaves of the Shepherdess". Eleusis 4 (April): 31–39. http://www.entheology.org/salvia-ott/ottonsalvia.htm. Retrieved 2009-04-04.
- Pendell, Dale (1995). "The Salvia divinorum chapter". Pharmako/Poeia: Plant Powers, Poisons, and Herbcraft. San Francisco: Mercury House. ISBN 1-56279-069-2. http://www.sagewisdom.org/pharmakopoeia.html. Retrieved 2007-06-28.
- Peterson, Sen. Karen (2006). "Chapter 256—Formerly Senate Bill No 259 (aka "Brett's Law")". An Act to Amend Title 16 of the Delaware Code Relating to the Uniform Controlled Substances Act. The General Assembly of the State of Delaware. http://delcode.delaware.gov/sessionlaws/ga143/chp256.shtml. Retrieved 2007-10-16.
- Prisinzano, Thomas E. (2005). "Psychopharmacology of the hallucinogenic sage Salvia divinorum". Life Sciences 78 (5): 527–531. doi:10.1016/j.lfs.2005.09.008. PMID 16213533. http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T99-4H8FR4X-3&_coverDate=12%2F22%2F2005&_alid=512807284&_rdoc=1&_fmt=&_orig=search&_qd=1&_cdi=5109&_sort=d&view=c&_acct=C000010000&_version=1&_urlVersion=0&_userid=4385511&md5=95a834c7778d0c624c0ff7601272af62. Retrieved 2006-12-21.
- Prisinzano, Thomas; Kevin Tidgewell and Wayne W. Harding (2005). "k Opioids as potential treatments for stimulant dependence". The AAPS Journal (Springer New York) 7 (3): E592–E599. doi:10.1208/aapsj070361. ISSN 10.1208/aapsj070361. PMC 2751263. PMID 16353938. http://www.springerlink.com/content/p2v488h351uhwn67/.
- Przekop P, Lee T (2009). "Persistent psychosis associated with salvia divinorum use". The American Journal of Psychiatry 166 (7): 832. doi:10.1176/appi.ajp.2009.08121759. PMID 19570943. http://ajp.psychiatryonline.org/cgi/content/full/166/7/832.
- Reboletti, Rep. Dennis (January 2007). "Full Text of HB0457". Cont Sub-Salvia divinorum. Illinois General Assembly. http://www.ilga.gov/legislation/fulltext.asp?DocName=&SessionId=51&GA=95&DocTypeId=HB&DocNum=457&GAID=9&LegID=27398&SpecSess=&Session=. Retrieved 2007-10-16.
- Reboletti, Dennis (March 2007). "Reboletti Passes First Bill, Bans "Magic Mint"". Illinois State Representative Dennis M. Reboletti (R) 46th District. Archived from the original on 2007-06-08. http://www.webcitation.org/5PS7Z0JoH. Retrieved 2007-06-08.
- Reisfield, Aaron S. (1993). "The botany of Salvia divinorum (Labiatae)". SIDA, Contributions to Botany 15 (3): 349–366. http://www.sagewisdom.org/reisfield.html. Retrieved 2007-05-04.
- Reisfield, Aaron S. (1987). Systematic studies in Salvia L. (Lamiaceae) with special emphasis on subgenus Calosphace (Benth.) Benth. section Dusenostachys Epl.. University of Wisconsin - Madison. p. 199. http://minds.wisconsin.edu/handle/1793/11724?show=full. Retrieved 2009-04-05.
- Roth, Bryan L.; Baner, Karen; Westkaemper, Richard; Siebert, Daniel; Rice, Kenner C.; Steinberg, SeAnna; Ernsberger, Paul; Rothman, Richard B. (September 2002). "Salvinorin A: A potent naturally occurring nonnitrogenous kappa к opioid selective agonist". PNAS 99 (18): 11934–11939. doi:10.1073/pnas.182234399. PMC 129372. PMID 12192085. http://www.pnas.org/cgi/content/full/99/18/11934. Retrieved 2007-03-24.- supporting info table 1, table 2, table 3.
- Roth, Bryan L. (July 2007). "Fast Moving Fronts—Comments by Professor Bryan Roth". Essential Science Indicators (ESI). http://www.esi-topics.com/fmf/2007/july07-BryanRoth.html. Retrieved 2007-10-06.
- Rothman, RB; DA Gorelick & SJ Heishman et al. (April 2000). "An open-label study of a functional opioid kappa antagonist in the treatment of opioid dependence". Journal of Substance Abuse Treatment 18 (3): 277–281. doi:10.1016/S0740-5472(99)00074-4. PMID 10742642. http://opioids.com/kappa/index.html.
- Sak, Rep. Michael (February 2008). "House Bill 5700". Michigan Legislature. http://www.legislature.mi.gov/(S(x4svk3fwsekp4145gg2fdm55))/mileg.aspx?page=getObject&objectName=2008-HB-5700. Retrieved 2007-10-16. - Full Text of HB5700
- SAMHSA (February 2008). "The NSDUH Report: Use of Specific Hallucinogens: 2006". Substance Abuse and Mental Health Services Administration, Office of Applied Studies. http://www.oas.samhsa.gov/2k8/hallucinogens/hallucinogens.htm. Retrieved 2008-05-09.
- Schenk, S.; Partridge, B. (April 2001). "Effect of the kappa-opioid receptor agonist, U69593, on reinstatement of extinguished amphetamine self-administration behavior.". Pharmacol Biochem Behav. 68 (4): 629–34. doi:10.1016/S0091-3057(00)00478-0. PMID 11526958.
- Schultes, Richard Evans; Albert Hofmann (1992). Plants of the Gods: Their Sacred, Healing and Hallucinogenic Powers. Rochester, Vermont: Healing Arts Press. ISBN 0-89281-406-3. http://www.csp.org/chrestomathy/plants_of.html. Retrieved 2007-06-27.
- Seeman P, Guan HC, Hirbec H (August 2009). "Dopamine D2High receptors stimulated by phencyclidines, lysergic acid diethylamide, salvinorin A, and modafinil". Synapse 63 (8): 698–704. doi:10.1002/syn.20647. PMID 19391150.
- Shulgin, Dr. Alexander; Ann Shulgin (1997). "LSD-25". PiHKAL. Berkeley: Transform Press. ISBN 0-963-00969-9. http://www.erowid.org/library/books_online/tihkal/tihkal26.shtml. Retrieved 2008-09-12.
- Shulgin, Dr. Alexander (June 2003). "Ask Dr. Shulgin Online". Salvia Divinorum, Law, & Medicine. Center for Cognitive Liberty & Ethics. http://www.cognitiveliberty.org/shulgin/adsarchive/salvia_legal.htm. Retrieved 2007-10-14.
- Siebert, Daniel (June 1994). "Salvia divinorum and salvinorin A: new pharmacologic findings". Journal of Ethnopharmacology 43 (1): 53–56. doi:10.1016/0378-8741(94)90116-3. PMID 7526076.
- Siebert, Daniel (2002). "A Prominent Salvia Divinorum Researcher Speaks Out: Letter to Congress (RE: Bill H.R. 5607)". The Entheogens and Drug Policy Project. Center for Cognitive Liberty & Ethics. http://www.cognitiveliberty.org/drug_policy/Daniel_Siebert_salvia_letter.html. Retrieved 2007-10-16.
- Siebert, Daniel (2003). "The history of the first Salvia divinorum plants cultivated outside of Mexico". The Entheogen Review XII (4). http://www.sagewisdom.org/salviahistory.html. Retrieved 2009-11-28.
- Siebert, Daniel (2004). "Localization of Salvinorin A and Related Compounds in Glandular Trichomes of the Psychoactive Sage, Salvia divinorum". Annals of Botany (Oxford University Press) 93 (6): 763–71. doi:10.1093/aob/mch089. PMID 15087301. http://aob.oxfordjournals.org/cgi/content/abstract/mch089v1?ijkey=55zbQRbeCanlM&keytype=ref. Retrieved 2009-04-13.
- Siebert, Daniel. "How to Propagate and Grow Salvia divinorum". The Salvia divinorum Research and Information Center. http://www.sagewisdom.org/salvgrow.html. Retrieved 2009-11-16.
- Siebert, Daniel. "How to obtain effects from smoked Salvia divinorum". The Salvia divinorum Research and Information Center. http://www.sagewisdom.org/smokeadvice.html. Retrieved 2007-06-19.
- Siebert, Daniel. "The Salvia divinorum FAQ". The Salvia divinorum Research and Information Center. http://sagewisdom.org/faq.html. Retrieved 2007-07-05.
- Sage Student; Daniel Siebert. "The Salvia divinorum User's Guide". The Salvia divinorum Research and Information Center. http://www.sagewisdom.org/usersguide.html. Retrieved 2007-06-27.
- Siebert, Daniel. "Salvia Divinorum Inspired Arts". The Salvia divinorum Research and Information Center. http://www.sagewisdom.org/arts.html. Retrieved 2007-06-28.
- Siebert, Daniel. "The Legal Status of Salvia divinorum". The Salvia divinorum Research and Information Center. http://www.sagewisdom.org/legalstatus.html. Retrieved 2007-03-04.
- Singh, Sundeep (2006). "Case Report: Adolescent salvia substance abuse". Addiction (Society for the Study of Addiction) 102 (5): 823–824. doi:10.1111/j.1360-0443.2007.01810.x. PMID 17493110. http://www3.interscience.wiley.com/journal/117967934/abstract?CRETRY=1&SRETRY=0.
- Strain, Rep. Michael G. (2005). "Louisiana Law, Revised Statutes, Rule Number 40, Section Number 989.1 — Unlawful production, manufacture, distribution, or possession of hallucinogenic plants". The Louisiana State Legislature. http://www.legis.state.la.us/lss/lss.asp?doc=321523. Retrieved 2008-09-15.
- Sullum, Jacob (December 2009). "The Salvia Ban Wagon". Reason Magazine. http://reason.com/archives/2009/11/19/the-salvia-ban-wagon/singlepage. Retrieved 2009-12-16.
- Sutton, John (2004). The Gardener's Guide to Growing Salvias. Workman Publishing Company. p. 19. ISBN 9780881926712.
- Assemblymen Jack Conners and Herb Conaway (April 21, 2006). "Conners/Conaway to sponsor bill outlawing herbal hallucinogen Salvia divinorum: Potent 'Diviner's Sage' Plant Produces Powerful LSD-Like High; Legal Substance Attracts Teen-age Interest via Internet Sites" (press release). Retrieved from PolitickerNJ.com on July 21, 2009.
- Tidgewell, Kevin; Harding, Wayne; Holden, Ken; Dersch, Christina; Butelman, Eduardo; Rothman, Richard; Prisinzano, Thomas (September 2004). "Neoclerodane Diterpenes as Potential Drug Abuse Therapeutics". The AAPS Journal 6 (Theme issue:Drug Addiction—From Basic Research to Therapies (AAPS-NIDA Symposium Frontiers in Science)). Archived from the original on 2008-01-12. http://web.archive.org/web/20080120110808/http://www.aapspharmsci.org/theme_issues/abstracts/view.asp?art=Front-04-00022&pdf=yes. Retrieved 2007-05-20.
- Turner, D.M. (August 1996). "Effects and Experiences". Salvinorin—The Psychedelic Essence of Salvia Divinorum. San Francisco, CA: Panther Press. ISBN 0-9642636-2-9. http://www.erowid.org/library/books_online/salvinorin/fx.shtml. Retrieved 2007-05-20.
- Valdés, Leander J. III; Díaz, José Luis; Paul, Ara G. (May 1983). "Ethnopharmacology of ska María Pastora (Salvia divinorum, Epling and Játiva-M)". Journal of Ethnopharmacology 7 (3): 287–312. doi:10.1016/0378-8741(83)90004-1. PMID 6876852. http://www.salvia-divinorum-scotland.co.uk/salvia/ethnopharmacology.htm. Retrieved 2007-05-04.
- Valdés, Leander J. III; Hatfield, G. M.; Koreeda, M.; Paul, A. G. (May 1987). "Studies of Salvia divinorum (Lamiaceae), an Hallucinogenic Mint from the Sierra Mazateca in Oaxaca, Central Mexico". Economic Botany 41 (2): 283–291. doi:10.1007/BF02858975. http://www.sagewisdom.org/valdes87.html. Retrieved 2009-04-05.
- Valdés, Leander J. III (2001). "The Early History of Salvia divinorum". The Entheogen Review X (1): 73–75. http://www.sagewisdom.org/earlysdhistory.html. Retrieved 2007-08-16.
- Wasson, R. Gordon (November 1963). "Notes on the Present Status of Ololiuhqui and the Other Hallucinogens of Mexico". Botanical Museum Leaflets, Harvard University 20 (6): 161–212. http://pages.prodigy.com/GBonline/liquix.htm. Retrieved 2007-09-15.
- YouTube (2008). "YouTube Community Guidelines". YouTube. http://www.youtube.com/t/community_guidelines. Retrieved 2008-09-17.
- Zhang, Yong; Butelman, Eduardo R.; Schlussman, Stefan D.; Ho, Ann; Kreek, Mary Jeanne (May 2005). "Effects of the plant-derived hallucinogen salvinorin A on basal dopamine levels in the caudate putamen and in a conditioned place aversion assay in mice: agonist actions at κ-Opioid receptors". Psychopharmacology 179 (3): 551–558. doi:10.1007/s00213-004-2087-0. PMID 15682306. http://www.springerlink.com/content/2e2e65wvctqbenvl/. Retrieved 2007-05-04.
News references
UK
- Doward, Jamie (2009-04-26). "There are many drugs that help people get out of their minds yet stay within the law - they're called 'legal highs'.". The Observer. http://www.guardian.co.uk/politics/2009/apr/26/drugs-legal-substances-highs. Retrieved 2009-04-26.
- Sherwell, Philip (2009-04-06). "Salvia: more powerful than LSD, and legal". The Daily Telegraph. http://www.telegraph.co.uk/health/healthnews/5090078/Salvia-more-powerful-than-LSD-and-legal.html. Retrieved 2009-04-17.
- Rohrer, Finlo (2007-11-01). "The mystery sage". BBC News. http://news.bbc.co.uk/1/hi/magazine/7071010.stm. Retrieved 2007-11-01.
- Vince, Gaia (2006-09-29). "Mind-altering drugs: does legal mean safe?". New Scientist. http://www.newscientist.com/channel/being-human/mg19125711.000-legal-highs-on-the-rise.html. Retrieved 2006-09-29.
- "Legal, but this is no party drug says net". Worksop Today. 2005-10-14. http://www.worksoptoday.co.uk/ViewArticle2.aspx?SectionID=741&ArticleID=1222314. Retrieved 2005-07-17.[dead link]
US
- Michael, Jenny (2009-04-22). "Man gets deferred sentence for salvia charge". Bismarck Tribune. http://www.bismarcktribune.com/articles/2009/04/22/news/local/183138.txt. Retrieved 2009-04-22. North Dakota.
- Honeycutt Spears, Valarie (2009-02-09). "Proposal would outlaw hallucinogenic plant salvia". www.kentucky.com. http://www.kentucky.com/181/story/688321.html. Retrieved 2009-02-09. Kentucky.
- "Salvia Divinorum: Nebraska Man is Acquitted of Sales Charge, But the Plant is Under Continued Attack There and Elsewhere". the Drug Reform Coordination Network (DRCNet). 2009-01-30. http://stopthedrugwar.org/chronicle/570/christian_firoz_salvia_nebraska_dakota_maryland_texas. Nebraska.
- Mason, Edward (2009-01-30). "City politician seeks to snuff out fad". The Boston Herald. Archived from the original on 2009-02-06. http://www.webcitation.org/5eO4uHqBh. Retrieved 2009-02-06.
- Michael, Sara (2009-01-28). "Lawmakers seek to make hallucinogenic herb illegal". The Baltimore Examiner. Archived from the original on 2009-02-06. http://www.webcitation.org/5eOd0fEhF. Retrieved 2009-02-06. Maryland.
- Sarno, David (2008-09-12). "Inspecting YouTube's ban on "drug abuse" videos". Los Angeles Times. http://latimesblogs.latimes.com/webscout/2008/09/inspecting-yout.html. Retrieved 2008-09-17.
- Sack, Kevin & Brent McDonald (2008-09-08). "Popularity of a Hallucinogen May Thwart Its Medical Uses". The New York Times. http://www.nytimes.com/2008/09/09/us/09salvia.html?pagewanted=1&ei=5124&en=aa0342b715969c4c&ex=1378699200&partner=permalink&exprod=permalink. Retrieved 2008-09-12.
- Michael, Jenny (2008-08-03). "Drug outlawed in North Dakota puts man on the spot". Bismarck Tribune. http://www.bismarcktribune.com/articles/2008/08/03/news/topnews/161483.txt. Retrieved 2008-08-25. North Dakota.
- Sege, Irene (2008-07-22). "Trying to outlaw an herbal high". The Boston Globe. http://www.boston.com/lifestyle/articles/2008/07/22/trying_to_outlaw_an_herbal_high/?page=2. Retrieved 2008-07-25. Massachusetts.
- Shafer, Jack (2008-05-06). "Salvia Divinorum Hysteria - The Press Helps Fuel The Next 'Drug Menace'". Slate Magazine/Washington Post. http://www.slate.com/id/2190781/pagenum/all/#page_start. Retrieved 2008-05-10.
- McNamara, Neal (2008-04-30). "A trip down salvia lane...". City Pulse - Lansing. http://www.lansingcitypulse.com/lansing/article-1844-a-trip-down-salvia-lane.html. Retrieved 2008-05-10. Michigan.
- "North Dakota Man Facing Years in Prison After Buying Salvia Divinorum On eBay". the Drug Reform Coordination Network (DRCNet). 2008-04-25. http://stopthedrugwar.org/chronicle/533/north_dakota_first_salvia_arrest_kenneth_rau. North Dakota.
- Chancellor, Aubrey Mika (2008-03-14). "Salvia Ban in San Antonio?". WOAI - News 4. http://www.woai.com/news/local/story.aspx?content_id=96d091b3-cf16-4e29-a829-1d520f62596f. Retrieved 2008-03-17.[dead link] Texas (+ viewer/reader feedback).
- Hoyle, Suzanne; Harris, Alexander (2008-03-12). "Salvia's herbal high spurs push for ban". The Miami Herald. Archived from the original on 2008-03-17. http://www.webcitation.org/5WNwt9uBs. Retrieved 2009-07-20. Florida (story includes online poll).
- White, Steve (2008-01-08). "Lawmakers Want to Ban YouTube Drug". Nebraska TV. http://www.nebraska.tv/Global/story.asp?S=7590482&nav=menu605_2. Retrieved 2008-01-08.
- Berry, Jeniffer (2008-01-07). "Salvia becomes new drug threat among teens". KHAS TV News 5. Archived from the original on 2008-01-10. http://www.webcitation.org/5UkWWUEsS. Retrieved 2008-01-08. Nebraska.
- Wallace, Todd (2007-11-26). "Indiana's Legal High: Teens Turned On To Powerful Drug". Indianapolis News (6News) (TheIndyChannel.com). http://www.theindychannel.com/news/14694672/detail.html. Retrieved 2007-11-27. Indiana (story includes online poll).
- Follow-up story: "Indiana's Legal High: Regulating Substance Faces Long Road". 2007-11-27. http://www.theindychannel.com/news/14703208/detail.html. Retrieved 2007-11-29.
- Moran, Terry & Max Culhane (2007-10-03). "Parents Blame Exotic Plant for Son's Suicide". Nightline (ABC News). http://abcnews.go.com/Nightline/Story?id=3685391&page=1. Retrieved 2007-10-06.
- Smith, Tracy (2007-09-25). "Mom Says Legal Herb Killed Son". CBS News. http://www.cbsnews.com/stories/2007/09/25/earlyshow/contributors/tracysmith/main3295276.shtml. Retrieved 2007-10-22.
- Viren, Sarah (2007-08-23). "'Magic mint' Salvia drug gains attention". Houston Chronicle. http://www.chron.com/CDA/archives/archive.mpl?id=2007_4410218. Retrieved 2009-07-20.
- Chalmers, Mike (2007-08-03). "Parents sue over dead son's salvia use". The News Journal (Delaware Online). Archived from the original on 2007-08-05. http://www.webcitation.org/5Qs62PkRg. Retrieved 2007-08-08.
- Baskin, Roberta (2007-07-11). "Exclusive I-Team Investigation of a hallucinogenic drug that has begun to sweep the nation". abc7News(WJLA-TV). Archived from the original on September 27, 2007. http://web.archive.org/web/20070927183108/http://news.wjla.com/news/stories/0707/438484.html. Retrieved 2007-07-13. Washington.
- Tompkins, Al (2007-07-13). "More Seeking Salvia, the Legal High (Q&A with WJLA-TV's Roberta Baskin)". The Poynter Institute. http://www.poynter.org/column.asp?id=2&aid=126587. Retrieved 2007-07-13.
- Allday, Erin (2007-06-27). "Legal, intense hallucinogen raises alarms". San Francisco Chronicle. http://www.sfgate.com/cgi-bin/article.cgi?file=/c/a/2007/06/27/NEWDRUG.TMP. Retrieved 2007-06-27. California.
- Martell, Chris (2007-06-18). "Herb is as potent as LSD". Wisconsin State Journal. http://www.madison.com/archives/read.php?ref=/wsj/2007/06/18/0706170218.php. Retrieved 2009-07-26. "newspaper's full front page (pdf, archived from the original) + related story link:Herb is as potent as LSD + WSJ reader's opinions (as published, archived from the original)."
- Rose, Sontaya (2007-05-10). "New High Sweeping Central Valley Teens". abc30ActionNews. Archived from the original on 2007-06-20. http://www.webcitation.org/5PkUNxM1n. Retrieved 2007-05-10. California.
- Eckenrode, Vicky (2007-03-08). "Ban on mind-altering herb weighed in legislature". Savannah Morning News. http://savannahnow.com/node/239324. Retrieved 2007-10-17. Georgia.
- Clark, Aaron (2007-03-05). "Oregon lawmakers consider banning legal hallucinogenic". The Worldlink/Associated Press. http://www.theworldlink.com/articles/2007/03/05/news/news12030507.txt. Retrieved 2007-10-16. Oregon.
- Masis, Julie (2007-02-28). "Mexican drug gains U.S. following". Reuters. Archived from the original on 2007-05-28. http://www.webcitation.org/5PAyYhkVU. Retrieved 2007-08-22. Boston.
- DeVine, Josh (2007-02-19). "New legal herb may do more damage than LSD". abc12(WJRT). http://abclocal.go.com/wjrt/story?section=local&id=5048914. Retrieved 2007-02-19. Michigan.
- Sanchick, Myra (2007-02-15). "Salvia: Underground Drug Getting Attention". Fox6News Milwaukee WITI-TV. Archived from the original on 2007-10-17. http://web.archive.org/web/20071017011636rn_1/www.myfoxmilwaukee.com/myfox/pages/Home/Detail?contentId=2395758&version=2&locale=EN-US&layoutCode=VSTY&pageId=1.1.1. Retrieved 2007-06-18.
- Sanchick, Myra (2007-02-14). "Salvia: The New Pot". Fox6News Milwaukee WITI-TV. Archived from the original on 2007-10-11. http://web.archive.org/web/20071011135135rn_1/www.myfoxmilwaukee.com/myfox/pages/Home/Detail?contentId=2385437&version=1&locale=EN-US&layoutCode=VSTY&pageId=1.1.1. Retrieved 2007-06-27.
- Haskell, Meg (2007-02-08). "Amended salvia bill limits sales". Bangor Daily News, Maine. Archived from the original on 2007-02-22. http://web.archive.org/web/20070222044942/http://bangordailynews.com/news/t/news.aspx?articleid=146136&zoneid=500. Retrieved 2007-10-16.
- "Lawmakers hear about a new drug". KXMBTV. 2007-01-31. http://www.kxma.com/getARticle.asp?ArticleId=91151. Retrieved 2007-07-17.
- Haskell, Meg (2007-01-23). "Lawmakers hear arguments on salvia ban". Bangor Daily News. Archived from the original on 2007-02-20. http://web.archive.org/web/20070220023714/http://bangordailynews.com/news/t/news.aspx?articleid=145495&zoneid=500. Retrieved 2007-07-16.
- Quinones, Todd (2006-11-30). "Deadly Dangers Of A Street Legal High". CBS 3 Philadelphia. Archived from the original on 2007-12-30. http://web.archive.org/web/20071230201450rn_1/cbs3.com/specialreports/Salvia.Divinorum.Legal.2.304828.html. Retrieved 2007-07-17.
- Dujanovic, Debbie (2006-11-30). "Dangerous Herb is Legal in Utah". KSL. http://www.ksl.com/?nid=148&sid=679822. Retrieved 2007-07-17.
- Follow-up story: Lawmaker Responds to Investigative Report on Dangerous Herb, 2006-11-28.
- Cardall, Duane. KSL Editorial, 2006-12-01.
- Blake, Katherine (2006-11-13). "DEA Warns Over-The-Counter Drug Is Like Acid". CBS 4 Denver. Archived from the original on 2007-05-28. http://www.webcitation.org/query?id=1180364376227947. Retrieved 2007-06-27.
- Chalmers, Mike (2006-05-06). "Salvia's Banned, but now the tough part.". The News Journal (Delaware Online). http://www.salvia.net/articles.php?id=3. Retrieved 2007-10-14.
- Cooper, Anderson (2006-04-13). "Salvia: Legal but Lethal". CNN. http://transcripts.cnn.com/TRANSCRIPTS/0604/13/lol.03.html. Retrieved 2007-07-17.
- viewer feedback—asx video (save & use media player)
- "Cheap, Legal And Dangerous—Salvia Hits Area". NBC10. 2006-04-11. Archived from the original on 200-01-05. http://web.archive.org/web/20080105212407/http://www.nbc10.com/news/8628557/detail.html. Retrieved 2007-10-22.
- Schaper, David (2006-03-20). <- in case original URL dies --> "Scientists See Research Value in Salvia". NPR (National Public Radio). Archived from the original on 2007-10-06 -->. http://www.webcitation.org/5SOEbcpsu <- in case original URL dies -->. Retrieved 2007-10-06. Broadcast transcript.
- Jones, Richard Lezin (2001-06-09). "New Cautions Over a Plant With a Buzz". The New York Times. http://query.nytimes.com/gst/fullpage.html?res=9401EEDA1038F93AA35754C0A9679C8B63. Retrieved 2007-12-11.
Further research
- Epling, Carl; Játiva-M, Carlos D. (December 1962). "A New Species of Salvia from Mexico". Botanical Museum Leaflets, Harvard University 20 (3). http://www.sagewisdom.org/epling&jativa.html. Retrieved 2007-05-08.
- Hanes, Karl R. (Spring 2003). "Salvia divinorum: Clinical and Research Potential". Multidisciplinary Association for Psychedelic Studies 13 (1): 18–20. http://www.maps.org/news-letters/v13n1/13118han.html. Retrieved 2007-05-19.
- Hofmann, Albert (1980). "Chapter 6. The Mexican Relatives of LSD". LSD—My Problem Child. New York: McGraw-Hill. ISBN 0-07-029325-2.
- Sherratt, Dr Andrew (Presenter); Sarah Marris (Producer); with Daniel Seibert, Dr Françoise Barbira-Freedman, Dr Tim Kendell, Dr Jon Robbins and Sean Thomas (1998) (video). Sacred Weeds: Salvia divinorum (Documentary). UK: TVF Productions (for Channel 4). http://video.google.com/videoplay?docid=4829797616419921428. Retrieved 2007-08-08.
- Wasson, R. Gordon (December 1962). "A New Mexican Psychotropic Drug from the Mint Family". Botanical Museum Leaflets, Harvard University 20 (3): 53–56. http://www.sagewisdom.org/wasson1.html. Retrieved 2007-05-06.
Dopaminergics Receptor ligands AgonistsAdamantanes: Amantadine • Memantine • Rimantadine; Aminotetralins: 7-OH-DPAT • 8-OH-PBZI • Rotigotine • UH-232; Benzazepines: 6-Br-APB • Fenoldopam • SKF-38,393 • SKF-77,434 • SKF-81,297 • SKF-82,958 • SKF-83,959; Ergolines: Bromocriptine • Cabergoline • Dihydroergocryptine • Lisuride • LSD • Pergolide; Dihydrexidine derivatives: 2-OH-NPA • A-86,929 • Ciladopa • Dihydrexidine • Dinapsoline • Dinoxyline • Doxanthrine; Others: A-68,930 • A-77,636 • A-412,997 • ABT-670 • ABT-724 • Aplindore • Apomorphine • Aripiprazole • Bifeprunox • BP-897 • CY-208,243 • Dizocilpine • Etilevodopa • Flibanserin • Ketamine • Melevodopa • Modafinil • Pardoprunox • Phencyclidine • PD-128,907 • PD-168,077 • PF-219,061 • Piribedil • Pramipexole • Propylnorapomorphine • Pukateine • Quinagolide • Quinelorane • Quinpirole • RDS-127 • Ro10-5824 • Ropinirole • Rotigotine • Roxindole • Salvinorin A • SKF-89,145 • Sumanirole • Terguride • Umespirone • WAY-100,635AntagonistsTypical antipsychotics: Acepromazine • Azaperone • Benperidol • Bromperidol • Clopenthixol • Chlorpromazine • Chlorprothixene • Droperidol • Flupentixol • Fluphenazine • Fluspirilene • Haloperidol • Loxapine • Mesoridazine • Methotrimeprazine • Nemonapride • Penfluridol • Perazine • Periciazine • Perphenazine • Pimozide • Prochlorperazine • Promazine • Sulforidazine • Sulpiride • Sultopride • Thioridazine • Thiothixene • Trifluoperazine • Triflupromazine • Trifluperidol • Zuclopenthixol; Atypical antipsychotics: Amisulpride • Asenapine • Blonanserin • Cariprazine • Carpipramine • Clocapramine • Clozapine • Gevotroline • Iloperidone • Lurasidone • Melperone • Molindone • Mosapramine • Olanzapine • Paliperidone • Perospirone • Piquindone • Quetiapine • Remoxipride • Risperidone • Sertindole • Tiospirone • Ziprasidone • Zotepine; Antiemetics: AS-8112 • Alizapride • Bromopride • Clebopride • Domperidone • Metoclopramide • Thiethylperazine; Others: Amoxapine • Buspirone • Butaclamol • Ecopipam • EEDQ • Eticlopride • Fananserin • L-745,870 • Nafadotride • Nuciferine • PNU-99,194 • Raclopride • Sarizotan • SB-277,011-A • SCH-23,390 • SKF-83,959 • Sonepiprazole • Spiperone • Spiroxatrine • Stepholidine • Tetrahydropalmatine • Tiapride • UH-232 • YohimbineReuptake inhibitors PlasmalemmalDAT inhibitorsPiperazines: DBL-583 • GBR-12,935 • Nefazodone • Vanoxerine; Piperidines: BTCP • Desoxypipradrol • Dextromethylphenidate • Difemetorex • Ethylphenidate • Methylnaphthidate • Methylphenidate • Phencyclidine • Pipradrol; Pyrrolidines: Diphenylprolinol • Methylenedioxypyrovalerone (MDPV) • Naphyrone • Prolintane • Pyrovalerone; Tropanes: β-CPPIT • Altropane • Brasofensine • CFT • Cocaine • Dichloropane • Difluoropine • FE-β-CPPIT • FP-β-CPPIT • Ioflupane (123I) • Iometopane • RTI-112 • RTI-113 • RTI-121 • RTI-126 • RTI-150 • RTI-177 • RTI-229 • RTI-336 • Tenocyclidine • Tesofensine • Troparil • Tropoxane • WF-11 • WF-23 • WF-31 • WF-33; Others: Adrafinil • Armodafinil • Amfonelic acid • Amineptine • Benzatropine (Benztropine) • Bromantane • BTQ • BTS-74,398 • Bupropion (Amfebutamone) • Ciclazindol • Diclofensine • Dimethocaine • Diphenylpyraline • Dizocilpine • DOV-102,677 • DOV-21,947 • DOV-216,303 • Etybenzatropine (Ethylbenztropine) • EXP-561 • Fencamine • Fencamfamine • Fezolamine • GYKI-52,895 • Indatraline • Ketamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Mazindol • Medifoxamine • Mesocarb • Modafinil • Nefopam • Nomifensine • NS-2359 • O-2172 • Pridefrine • Propylamphetamine • Radafaxine • SEP-225,289 • SEP-227,162 • Sertraline • Sibutramine • Tametraline • Tedatioxetine • TripelennamineVMAT inhibitorsReleasing agents Morpholines: Fenbutrazate • Morazone • Phendimetrazine • Phenmetrazine; Oxazolines: 4-Methylaminorex (4-MAR, 4-MAX) • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone; Phenethylamines (also amphetamines, cathinones, phentermines, etc): 2-Hydroxyphenethylamine (2-OH-PEA) • 4-CAB • 4-Methylamphetamine (4-MA) • 4-Methylmethamphetamine (4-MMA) • Alfetamine • Amfecloral • Amfepentorex • Amfepramone • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • β-Methylphenethylamine (β-Me-PEA) • Benzodioxolylbutanamine (BDB) • Benzodioxolylhydroxybutanamine (BOH) • Benzphetamine • Buphedrone • Butylone • Cathine • Cathinone • Clobenzorex • Clortermine • D-Deprenyl • Dimethoxyamphetamine (DMA) • Dimethoxymethamphetamine (DMMA) • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, metamfepramone) • Ethcathinone (Ethylpropion) • Ethylamphetamine • Ethylbenzodioxolylbutanamine (EBDB) • Ethylone • Famprofazone • Fenethylline • Fenproporex • Flephedrone • Fludorex • Furfenorex • Hordenine • Lophophine (Homomyristicylamine) • Mefenorex • Mephedrone • Methamphetamine (Desoxyephedrine, Methedrine; Dextromethamphetamine, Levomethamphetamine) • Methcathinone (Methylpropion) • Methedrone • Methoxymethylenedioxyamphetamine (MMDA) • Methoxymethylenedioxymethamphetamine (MMDMA) • Methylbenzodioxolylbutanamine (MBDB) • Methylenedioxyamphetamine (MDA, tenamfetamine) • Methylenedioxyethylamphetamine (MDEA) • Methylenedioxyhydroxyamphetamine (MDOH) • Methylenedioxymethamphetamine (MDMA) • Methylenedioxymethylphenethylamine (MDMPEA, homarylamine) • Methylenedioxyphenethylamine (MDPEA, homopiperonylamine) • Methylone • Ortetamine • Parabromoamphetamine (PBA) • Parachloroamphetamine (PCA) • Parafluoroamphetamine (PFA) • Parafluoromethamphetamine (PFMA) • Parahydroxyamphetamine (PHA) • Paraiodoamphetamine (PIA) • Paredrine (Norpholedrine, Oxamphetamine) • Phenethylamine (PEA) • Pholedrine • Phenpromethamine • Prenylamine • Propylamphetamine • Tiflorex (Flutiorex) • Tyramine (TRA) • Xylopropamine • Zylofuramine; Piperazines: 2,5-Dimethoxy-4-bromobenzylpiperazine (2C-B-BZP) • Benzylpiperazine (BZP) • Methoxyphenylpiperazine (MeOPP, paraperazine) • Methylbenzylpiperazine (MBZP) • Methylenedioxybenzylpiperazine (MDBZP, piperonylpiperazine); Others: 2-Amino-1,2-dihydronaphthalene (2-ADN) • 2-Aminoindane (2-AI) • 2-Aminotetralin (2-AT) • 4-Benzylpiperidine (4-BP) • 5-IAI • Clofenciclan • Cyclopentamine • Cypenamine • Cyprodenate • Feprosidnine • Gilutensin • Heptaminol • Hexacyclonate • Indanylaminopropane (IAP) • Indanorex • Isometheptene • Methylhexanamine • Naphthylaminopropane (NAP) • Octodrine • Phthalimidopropiophenone • Propylhexedrine (Levopropylhexedrine) • Tuaminoheptane (Tuamine)Enzyme inhibitors PAH inhibitors3,4-DihydroxystyreneTH inhibitorsNonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • L-Deprenyl (Selegiline) • Ladostigil • Lazabemide • Milacemide • Pargyline • Rasagiline • SafinamideDBH inhibitorsOthers L-Phenylalanine → L-Tyrosine → L-DOPA (Levodopa)Ferrous iron (Fe2+) • Tetrahydrobiopterin • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal phosphate) • Vitamin B9 (Folic acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersActivity enhancers: Benzofuranylpropylaminopentane (BPAP) • Phenylpropylaminopentane (PPAP); Toxins: Oxidopamine (6-Hydroxydopamine)List of dopaminergic drugsOpioids Opium and
poppy straw
derivativesCrude opiate extracts/
whole opium productsCompote/Kompot/Polish heroin • Diascordium • B & O Supprettes • Dover's powder • Kendal Black Drop • Laudanum • Mithridate • Opium • Paregoric • Poppy straw concentrate • Poppy tea • Smoking opium • Theriac
Natural opiates Opium alkaloids
see also: Components of opiumCodeine • Morphine • Oripavine • Pseudomorphine • Thebaine
Alkaloid salts mixtures Semisynthetics
including
Bentley compoundsMorphine family 3,6-diesters of morphine Acetylpropionylmorphine • Diacetyldihydromorphine (Dihydroheroin, Acetylmorphinol) • Dibenzoylmorphine • Dipropanoylmorphine • Heroin (Diacetylmorphine) • Nicomorphine
Codeine-dionine family Morphinones and morphols 14-Cinnamoyloxycodeinone • 14-Ethoxymetopon • 14-Methoxymetopon • 14-Phenylpropoxymetopon • 7-Spiroindanyloxymorphone • Acetylmorphone • Codeinone • Codorphone • Codoxime • Thebacon (Acetyldihydrocodeinone / Dihydrocodeinone enol acetate) • Hydrocodone • Hydromorphone • Metopon • Morphinone • N-Phenethyl-14-ethoxymetopon • Oxycodone • Oxymorphol • Oxymorphone • Pentamorphone • Semorphone
Morphides Dihydrocodeine series 14-hydroxydihydrocodeine • Acetyldihydrocodeine • Dihydrocodeine • Nicocodeine • Nicodicodeine
Nitrogen morphine derivatives Hydrazones Halogenated morphine derivatives 1-Iodomorphine
Active opiate
metabolitesMorphine-6-glucuronide • 6-Monoacetylmorphine • Norcodeine • Normorphine
Morphinans Morphinan series Cyclorphan • Dextrallorphan • Levorphanol • Levophenacylmorphan • Levomethorphan • Norlevorphanol • Oxilorphan • Phenomorphan • Methorphan / Racemethorphan • Morphanol / Racemorphanol • Ro4-1539 • Xorphanol
Others Benzomorphans 8-Carboxamidocyclazocine • Alazocine • Bremazocine • Dezocine • Ketazocine • Metazocine • Pentazocine • Phenazocine
4-Phenylpiperidines Pethidines
(Meperidines)4-Fluoromeperidine • Allylnorpethidine • Anileridine • Benzethidine • Carperidine • Difenoxin • Diphenoxylate • Etoxeridine (Carbetidine) • Furethidine • Hydroxypethidine (Bemidone) • Morpheridine • Oxpheneridine (Carbamethidine) • Pethidine (Meperidine) • Pethidine Intermediate A • Pethidine Intermediate B (Norpethidine) • Pethidine Intermediate C (Pethidinic Acid) • Pheneridine • Phenoperidine • Piminodine • Properidine (Ipropethidine) • Sameridine
Prodines Ketobemidones Others Open chain
opioidsAmidones Methadols Moramides Dextromoramide • Levomoramide • Racemoramide
Thiambutenes Phenalkoxams Ampromides Others IC-26 • Isoaminile • Lefetamine • R-4066
Anilidopiperidines 3-Allylfentanyl • 3-Methylfentanyl • 3-Methylthiofentanyl • 4-Phenylfentanyl • Alfentanil • α-methylacetylfentanyl • α-methylfentanyl • α-methylthiofentanyl • β-hydroxyfentanyl • β-hydroxythiofentanyl • β-methylfentanyl • Brifentanil • Carfentanil • Fentanyl • Lofentanil • Mirfentanil • Ocfentanil • Ohmefentanyl • Parafluorofentanyl • Phenaridine • Remifentanil • Sufentanil • Thiofentanyl • Trefentanil
Oripavine
derivativesPhenazepanes Pirinitramides Benzimidazoles Indoles Diphenylmethylpiperazines Opioid peptides
see also: Opioid neuropeptidesAdrenorphin • Amidorphin • Casomorphin • DADLE • DAMGO • Dermorphin • Dynorphin • Endomorphin • Endorphins • Enkephalin • Gliadorphin • Morphiceptin • Nociceptin • Octreotide • Opiorphin • Rubiscolin • TRIMU 5
Others AD-1211 • AH-7921 • Azaprocin • BDPC • BRL-52537 • Bromadoline • C-8813 • Ciramadol • Doxpicomine • Enadoline • Faxeladol • GR-89696 • Herkinorin • ICI-199,441 • ICI-204,448 • J-113,397 • JTC-801 • Ketamine • LPK-26 • Methopholine • MT-45 • N-Desmethylclozapine • NNC 63-0532 • Nortilidine • O-Desmethyltramadol • Phencyclidine • Prodilidine • Profadol • Ro64-6198 • Salvinorin A • SB-612,111 • SC-17599 • RWJ-394,674 • TAN-67 • Tapentadol • Tifluadom • Tilidine • Tramadol • Trimebutine • U-50,488 • U-69,593 • Viminol • W-18
Opioid antagonists
&
inverse agonists5'-Guanidinonaltrindole • Alvimopan • Chlornaltrexamine • Cyclazocine • Cyprodime • Diprenorphine (M5050) • JDTic • Levallorphan • Methylnaltrexone • Nalfurafine • Nalmefene • Naloxazone • Naloxonazine • Naloxone • Nalorphine • Naltrexol-d4 • Naltrexone • Naltriben • Naltrindole • Norbinaltorphimine • Oxilorphan
List of opioidsCategories:- Entheogens
- Flora of Mexico
- Psychedelic drugs
- Psychoactive drugs
- Salvia
- Herbal and fungal hallucinogens
Wikimedia Foundation. 2010.