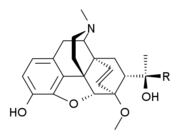
- Oripavine
-
Oripavine  6,7,8,14-Tetradehydro-4,5α-epoxy-
6,7,8,14-Tetradehydro-4,5α-epoxy-
6-methoxy-17-methylmorphinan-3-olOther names3-O-demethyl-thebaineIdentifiers CAS number 467-04-9 PubChem 5462306 ChemSpider 4575366 
EC number 207-385-6 KEGG C06175 
MeSH Oripavine ChEMBL CHEMBL437602 
ATC code N02 Jmol-3D images Image 1 - Oc5ccc4c2c5O[C@H]3C(/OC)=C\C=C1\[C@H](N(CC[C@]123)C)C4
Properties Molecular formula C18H19NO3 Molar mass 297.35 g mol−1 Pharmacology Routes of
administrationSC Legal status  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Oripavine is an opiate and the major metabolite of thebaine. It is the parent compound from which a series of semi-synthetic opioids are derived, which includes the compounds etorphine and buprenorphine. Although its analgesic potency is comparable to morphine, it is not used clinically due to its severe toxicity and low therapeutic index.
Contents
Pharmacological properties
Oripavine possesses an analgesic potency comparable to morphine; however, it is not clinically useful due to severe toxicity and low therapeutic index. In both mice and rats, toxic doses caused tonic-clonic seizures followed by death, similar to thebaine.[1] Oripavine has a potential for dependence which is significantly greater than that of thebaine but slightly less than that of morphine.[2]
Bridged Oripavine derivatives
Of much greater relevance are the properties of the orvinols, a large family of semi-synthetic oripavine derivatives classically synthesised by the Diels-Alder reaction of thebaine with an appropriate dienophile followed by 3-O-demethylation to the corresponding bridged oripavine. These compounds were developed by the group led by K. W. Bentley in the 1960s, and these Bentley compounds represent the first series of "super-potent" μ-opioid agonists, with some compounds in the series being over 10,000 times the potency of morphine as an analgesic.[3][4][5] The simple bridged oripavine parent compound 6,14-Endoethenotetrahydrooripavine is already 40x the potency of morphine,[6] but adding a branched tertiary alcohol substituent on the C7 position results in a wide range of highly potent compounds.[7]
Drug name R Analgesic Potency (Morphine = 1) methyl 63 ethyl 330 Etorphine n-propyl 3200 n-butyl 5200 isobutyl 10 n-pentyl 4500 isopentyl 9200 n-hexyl 58 O-desmethyl-7-PET phenethyl 2200 phenyl 34 cyclopentyl 70 cyclohexyl 3400 Other notable derivatives then result from further modification of this template, with saturation of the 7,8-double bond of etorphine resulting in the even more potent dihydroetorphine (up to 12,000x potency of morphine) and acetylation of the 3-hydroxy group of etorphine resulting in acetorphine (8700x morphine) - although while the isopentyl homologue of etorphine is nearly three times more potent, its 7,8-dihydro and 3-acetyl derivatives are less potent than the corresponding derivatives of etorphine at 11000x and 1300x morphine respectively. Replacing the N-methyl group with cyclopropylmethyl results in opioid antagonists such as diprenorphine (which is used as an antidote to reverse the effects of etorphine), and partial agonists such as buprenorphine, which is widely used in the treatment of opioid addiction.
Legal status
Due to the relative ease of synthetic modification of oripavine to produce other narcotics (by either direct or indirect routes via thebaine), the World Health Organization's Expert Committee on Drug Dependence recommended in 2003 that oripavine be controlled under Schedule I of the 1961 Single Convention on Narcotic Drugs.[8] On March 14, 2007, the United Nations Commission on Narcotic Drugs formally decided to accept these recommendations, and placed oripavine in the Schedule I.[9]
Until recently, oripavine was a Schedule II drug in the United States by default as a thebaine derivative, although it was not explicitly listed. However, as a member state under the 1961 Single Convention on Narcotic Drugs, the US was obliged to specifically control the substance under the Controlled Substances Act following its international control by the UN Commission on Narcotic Drugs. On September 24, 2007, the Drug Enforcement Administration formally added oripavine to Schedule II.[10]
References
- ^ Yeh, SY (December 1981). "Analgesic activity and toxicity of oripavine and phi-dihydrothebaine in the mouse and rat". Archives Internationales de Pharmacodynamie et de Therapie 254 (2): p223–40. PMID 6121539.
- ^ Chanoit, Pierre; et al. (1981). "Dependence potential of oripavine". Bulletin on Narcotics (WHO Advisory Group) 33 (3): 29–35. PMID 7039748. http://www.unodc.org/unodc/en/data-and-analysis/bulletin/bulletin_1981-01-01_3_page004.html. Retrieved 2007-10-05.
- ^ Bentley KW, Boura AL, Fitzgerald AE, Hardy DG, McCoubrey A, Aikman ML, Lister RE. Compounds Possessing Morphine-Antagonising or Powerful Analgesic Properties. Nature. 1965 Apr 3;206:102-3. PMID 14334338
- ^ Bentley KW, Hardy DG, Meek B. Novel analgesics and molecular rearrangements in the morphine-thebaine group. II. Alcohols derived from 6,14-endo-etheno- and 6,14-endo-ethanotetrahydrothebaine. Journal of the American Chemical Society. 1967 Jun 21;89(13):3273-80. PMID 6042763
- ^ Bentley KW, Hardy DG, Meek B. Novel analgesics and molecular rearrangements in the morphine-thebaine group. IV. Acid-catalyzed rearrangements of alcohols of the 6,14-endo-ethenotetrahydrothebaine series. Journal of the American Chemical Society. 1967 Jun 21;89(13):3293-303. PMID 6042765
- ^ Lewis JW, Bentley KW, Cowan A. Narcotic analgesics and antagonists. Annual Reviews in Pharmacology. 1971;11:241-70. PMID 4948499
- ^ Bentley KW, Hardy DG. Novel analgesics and molecular rearrangements in the morphine-thebaine group. III. Alcohols of the 6,14-endo-ethenotetrahydrooripavine series and derived analogs of N-allylnormorphine and -norcodeine. Journal of the American Chemical Society. 1967 Jun 21;89(13):3281-92. PMID 6042764
- ^ WHO Expert Committee on Drug Dependence. "Thirty-third report". WHO Technical Report Series, No. 915. Geneva, World Health Organization, 2003. Accessed September 17, 2007.
- ^ UN Commission on Narcotic Drugs. "Decision 50/1: Inclusion of oripavine in Schedule I of the Single Convention on Narcotic Drugs of 1961 and that Convention as amended by the 1972 Protocol." Report on the fiftieth session. Document E/CN.7/2007/16, p 52. Geneva, United Nations Office on Drugs and Crime, 2007. Accessed September 18, 2007.
- ^ Drug Enforcement Administration. "Designation of Oripavine as a Basic Class of Controlled Substance." Federal Register. September 2007; 72 (184):p54208-54210. Accessed October 25, 2007.
Categories:- Poisons
- Oripavines
- Opiates
- Phenol ethers
- Morphinans
Wikimedia Foundation. 2010.