- Levopropoxyphene
-
Levopropoxyphene 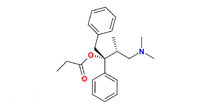 (1R,2S)-1-benzyl-3-(dimethylamino)-2-methyl-1-phenylpropyl propionateOther names[(2R,3S)-4-dimethylamino-3-methyl-1,2-diphenyl-butan-2-yl]propanoate
(1R,2S)-1-benzyl-3-(dimethylamino)-2-methyl-1-phenylpropyl propionateOther names[(2R,3S)-4-dimethylamino-3-methyl-1,2-diphenyl-butan-2-yl]propanoateIdentifiers CAS number 2338-37-6 
PubChem 200742 ChemSpider 173777 
ChEMBL CHEMBL1201241 
Jmol-3D images Image 1 - O=C(O[C@](c1ccccc1)(Cc2ccccc2)[C@@H](C)CN(C)C)CC
- InChI=1S/C22H29NO2/c1-5-21(24)25-22(18(2)17-23(3)4,20-14-10-7-11-15-20)16-19-12-8-6-9-13-19/h6-15,18H,5,16-17H2,1-4H3/t18-,22+/m0/s1

Key: XLMALTXPSGQGBX-PGRDOPGGSA-N
InChI=1/C22H29NO2/c1-5-21(24)25-22(18(2)17-23(3)4,20-14-10-7-11-15-20)16-19-12-8-6-9-13-19/h6-15,18H,5,16-17H2,1-4H3/t18-,22+/m0/s1
Key: XLMALTXPSGQGBX-PGRDOPGGBE
Properties Molecular formula C22H29NO2 Molar mass 339.47 g mol−1  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Levopropoxyphene is an antitussive. It is an optical isomer of dextropropoxyphene. The racemic mixture is called propoxyphene. Only the dextro-isomer (dextropropoxyphene) has an analgesic effect; the levo-isomer appears to exert only an antitussive effect. It was formerly marketed in the U.S. by Eli Lilly under the tradename Novrad as an antitussive.[1][2]
References
- ^ Reference.MD: Propoxyphene napsylate
- ^ Lutje Spelberg; Jeffrey Harald (2003). "Enantioselective biocatalytic conversions of epoxides". Rijksuniversiteit Groningen. http://irs.ub.rug.nl/ppn/242581676.
This drug article relating to the respiratory system is a stub. You can help Wikipedia by expanding it.
