- Ohmefentanyl
-
Ohmefentanyl 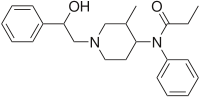
Systematic (IUPAC) name N-[(3R,4S)-1-[(2S)-2-hydroxy-2-phenyl-ethyl] -3-methyl-4-piperidyl]-N-phenyl-propanamide Clinical data Pregnancy cat. ? Legal status ? Identifiers CAS number 78995-14-9 
ATC code None PubChem CID 10474095 ChemSpider 8649506 
Chemical data Formula C23H30N2O2 Mol. mass 366.497 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Ohmefentanyl (β-hydroxy-3-methylfentanyl) was discovered in 1995[1] and is an extremely potent analgesic drug which selectively binds to the µ-opioid receptor.
Ohmefentanyl is one of the most potent μ -receptor agonists known, comparable to super-potent opioids such as carfentanil and etorphine which are used for tranquilizing large animals such as elephants in veterinary medicine. In mouse studies, the most active isomer 3R,4S,βS-ohmefentanyl was 28 times more powerful as a painkiller than fentanyl, the chemical from which it is derived, and 6300 times more effective than morphine.[2]. Ohmefentanyl has three stereogenic centers and so has eight stereoisomers, which are named F9201-F9208. Researchers are studying the different pharmaceutical properties of these isomers.[3].
The 4"-fluoro analogue (i.e. substituted on the phenethyl ring) of the 3R,4S,βS isomer of ohmefentanyl is one of the most potent opioid agonists yet discovered, possessing an analgesic potency approximately 18,000-fold greater than morphine.[4] Other analogues with potency higher than that of ohmefentanyl itself include the 2'-fluoro derivative (i.e. substituted on the aniline phenyl ring), and derivatives where the N-propionyl group was replaced by N-methoxyacetyl or 2-furamide groups, or a carboethoxy group is added to the 4-position of the piperidine ring (the latter is listed as being up to 30,000 times more potent than morphine.)[5]
See also
References
- ^ Brine, George A.; Stark, Peter A.; Liu, Young; Carrol, F. Ivy; Singh, P.; et al. Journal of Medicinal Chemistry, 1995 , vol. 38, #9 p. 1547 - 1557
- ^ Guo, G. W.; He, Y.; Jin, W. Q.; Zou, Y.; Zhu, Y. C.; Chi, Z. Q. (2000). "Comparison of physical dependence of ohmefentanyl stereoisomers in mice". Life sciences 67 (2): 113–120. doi:10.1016/S0024-3205(00)00617-2. PMID 10901279.
- ^ Liu, Z.; He, Y.; Jin, W.; Chen, X.; Shen, Q.; Chi, Z. (2004). "Effect of chronic treatment of ohmefentanyl stereoisomers on cyclic AMP formation in Sf9 insect cells expressing human mu-opioid receptors". Life sciences 74 (24): 3001–3008. doi:10.1016/j.lfs.2003.10.027. PMID 15051423.
- ^ Yong, Z.; Hao, W.; Weifang, Y.; Qiyuan, D.; Xinjian, C.; Wenqiao, J.; Youcheng, Z. (2003). "Synthesis and analgesic activity of stereoisomers of cis-fluoro-ohmefentanyl". Die Pharmazie 58 (5): 300–302. PMID 12779044.
- ^ Brine GA, Carroll FI, Richardson-Leibert TM, Xu H, Rothman RB. Ohmefentanyl and its stereoisomers: Chemistry and Pharmacology. Current Medicinal Chemistry. 1997; 4(4):247-270.
External links

This analgesic-related article is a stub. You can help Wikipedia by expanding it.

