- O-Desmethyltramadol
-
O-Desmethyltramadol 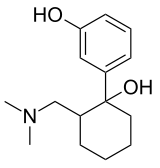
Systematic (IUPAC) name 3-[2-(1-Amino-1-methylethyl)-1-hydroxycyclohexyl]phenol Clinical data Pregnancy cat. ? Legal status ? (US) Routes Converted Metabolite Pharmacokinetic data Half-life ~ 9 h Identifiers CAS number 73986-53-5 
ATC code ? PubChem CID 130829 ChemSpider 115703 
ChEMBL CHEMBL1400 
Chemical data Formula C15H23NO2 Mol. mass 249.349 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)O-Desmethyltramadol is an opioid analgesic and the main active metabolite of tramadol.[1]
(+)-O-Desmethyltramadol is the most important metabolite of tramadol produced in the liver after tramadol is consumed. This metabolite is considerably more potent as a μ opioid agonist than the parent compound.[2]
Tramadol is demethylated by the liver enzyme CYP2D6[3] in the same way as codeine, and so similarly to the variation in effects seen with codeine, individuals who have a less active form of CYP2D6 ("poor metabolisers") will tend to get reduced analgesic effects from tramadol.
The two enantiomers of O-desmethyltramadol show quite distinct pharmacological profiles;[4] both (+) and (-)-O-desmethyltramadol are inactive as serotonin reuptake inhibitors,[5] but (-)-O-desmethyltramadol retains activity as a noradrenaline reuptake inhibitor[6] and so the mix of both the parent compound and metabolites produced contributes significantly to the complex pharmacological profile of tramadol.
References
- ^ Sevcik, J; Nieber, K; Driessen, B; Illes, P (1993). "Effects of the central analgesic tramadol and its main metabolite, O-desmethyltramadol, on rat locus coeruleus neurones". British journal of pharmacology 110 (1): 169–76. PMC 2175982. PMID 8220877. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2175982.
- ^ Dayer, P; Desmeules, J; Collart, L (1997). "Pharmacology of tramadol". Drugs 53 Suppl 2: 18–24. doi:10.2165/00003495-199700532-00006. PMID 9190321.
- ^ Borlak, J; Hermann, R; Erb, K; Thum, T (2003). "A rapid and simple CYP2D6 genotyping assay--case study with the analgetic tramadol". Metabolism: clinical and experimental 52 (11): 1439–43. doi:10.1016/S0026-0495(03)00256-7. PMID 14624403.
- ^ Garrido, MJ; Valle, M; Campanero, MA; Calvo, R; Trocóniz, IF (2000). "Modeling of the in vivo antinociceptive interaction between an opioid agonist, (+)-O-desmethyltramadol, and a monoamine reuptake inhibitor, (-)-O-desmethyltramadol, in rats". The Journal of pharmacology and experimental therapeutics 295 (1): 352–9. PMID 10992001.
- ^ Bamigbade, TA; Davidson, C; Langford, RM; Stamford, JA (1997). "Actions of tramadol, its enantiomers and principal metabolite, O-desmethyltramadol, on serotonin (5-HT) efflux and uptake in the rat dorsal raphe nucleus". British journal of anaesthesia 79 (3): 352–6. PMID 9389855.
- ^ Driessen, B; Reimann, W; Giertz, H (1993). "Effects of the central analgesic tramadol on the uptake and release of noradrenaline and dopamine in vitro". British journal of pharmacology 108 (3): 806–11. PMC 1908052. PMID 8467366. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1908052.
Categories:- Synthetic opioids
- Phenols
- Alcohols
- Amines
- Mu-opioid agonists
Wikimedia Foundation. 2010.
